3D Phenotypic Cellular Models
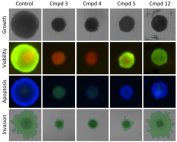
Growth Inhibition
Image and measure the size of 3D tumor spheroids under multiple drug and growth conditions over time.
Measure Apoptosis
Monitor apoptosis in 3D tumor spheroids.
Tumor Spheroid Viability
Detect and monitor the viability of 3D tumor spheroids.
Quantify Invasion into Matrigel
Measure the cell invasion from a spheroid into Matrigel.
Measure Migration onto ECM
Measure the cell outgrowth from a spheroid onto an extracellular matrix (ECM).
Tumorsphere Formation & Clonogenic Survival
Perform tumorsphere formation and clonogenic assays to quantify size and number of formed tumorspheres.
EBs & PDOs
Measure size and number of embryoid bodies and patient-derived organoids.
3D Confrontation Assay
Monitor the confrontation between 3D tumor spheroids.
Z-Stack & Volumetric Analysis
Acquire z-stack data from your cell samples and perform volumetric analysis with Revvity high-content screening systems.