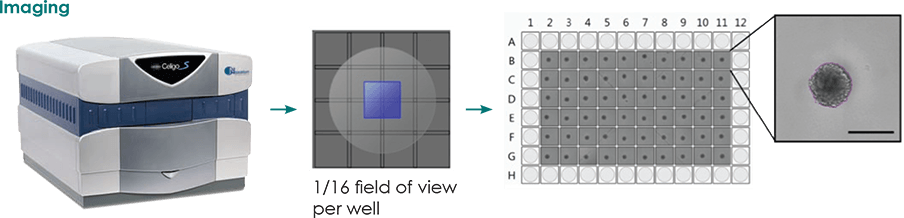
Monitor the Confrontation of 3D Tumor Spheroids
Monitor and Quantify the Confrontation of 3D Tumor Spheroids
- Directly image fluorescently labeled and bright field multicellular tumor spheroids
- Quantify the percent confrontation between two 3D spheroids
- Image the same plate over time to monitor spheroid confrontation
Introduction
The Celigo imaging cytometer has been developed to fully automate live cell analysis of multicellular tumor spheroids (MCTS). This automated morphometric analysis tool significantly reduces the time and effort needed to quantify key aspects of 3D spheres including size, growth, growth tracking over time, and response to chemotherapeutics.
Additionally, the Celigo software can determine the confluence ratio between two objects, providing an analysis measurement for researchers performing 3D confrontation assays.
Monitoring the Confrontation Between a 3D Astrocyte and a 3D Multicellular Tumor Spheroid
Experimental Setup

Celigo Time-Lapse Image Capture of a 3D Confrontation Assay Between an Astrocyte and a Multicellular Tumor Spheroid
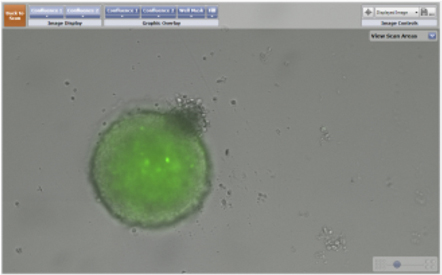
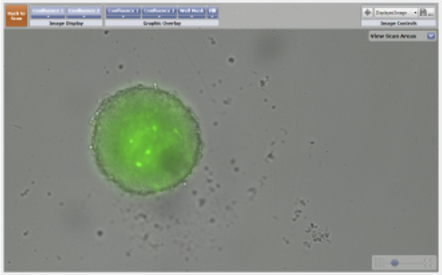
- The series of images below were taken over a 24 hour period to monitor confrontation between two 3D spheroids.
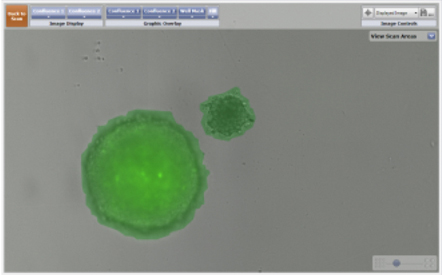
- The images on the left are of a GFP-labeled 3D multicellular tumor spheroid and a bright field image of a 3D astrocyte.
- The images on the right display the identification of both the 3D spheroid and the astrocyte using the Celigo confluence ratio algorithm. The identified objects are outlined with a green pseudocolor for easy identification.
Imaging of a GFP-labeled 3D tumor spheroid and a 3D astrocyte over a 24 hour period
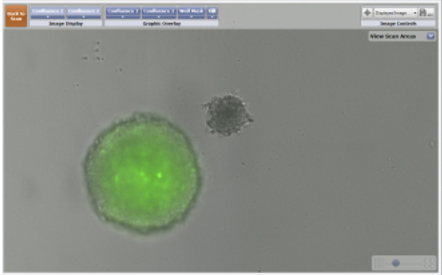
Imaging of a GFP-labeled 3D tumor spheroid and a 3D astrocyte over a 24 hour period
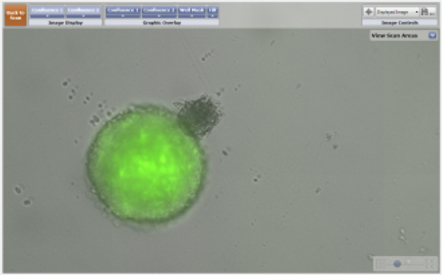



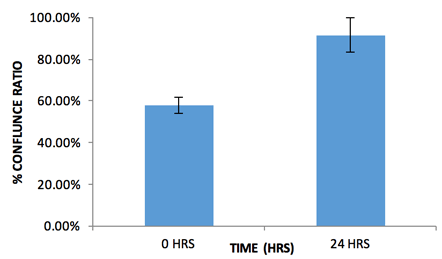
Measured Percent Confluence Ratio Between a GPF-Labeled 3D MCTS and a 3D Astrocyte
- The confluence ratio application calculates the ratio of the GFP confluence area that is within the confluence area of the bright field channel.
- In this case, the GFP signal from the 3D multicellular tumor spheroid occupies about 60% of the total measured bright field area (tumor spheroid + astrocyte). Over a 24-hour period, as the two spheroids merge, the total bright field area shrinks and the percent confluence ratio increases.
Imaging and Monitoring the Confrontation between a Multicellular Tumor Spheroid and an Embryoid Body
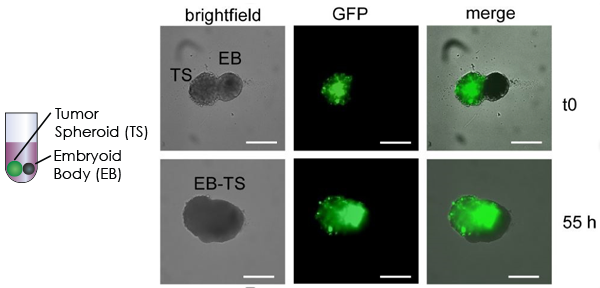
Similarly to the confrontation assay using a 3D astocyte, the Celigo was used to image the confrontation between a GFP-labeled MCTS and an EB over a 55-hour period.
