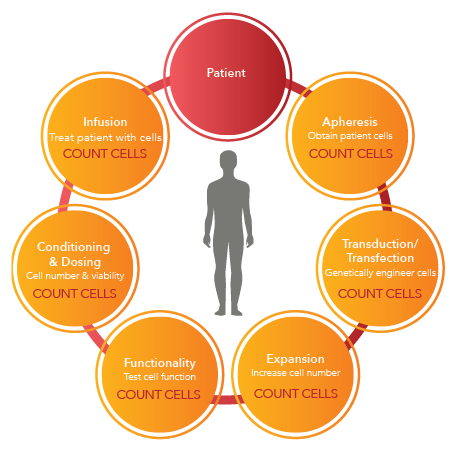
Accurate Cell Counting Methods for CAR T Therapy
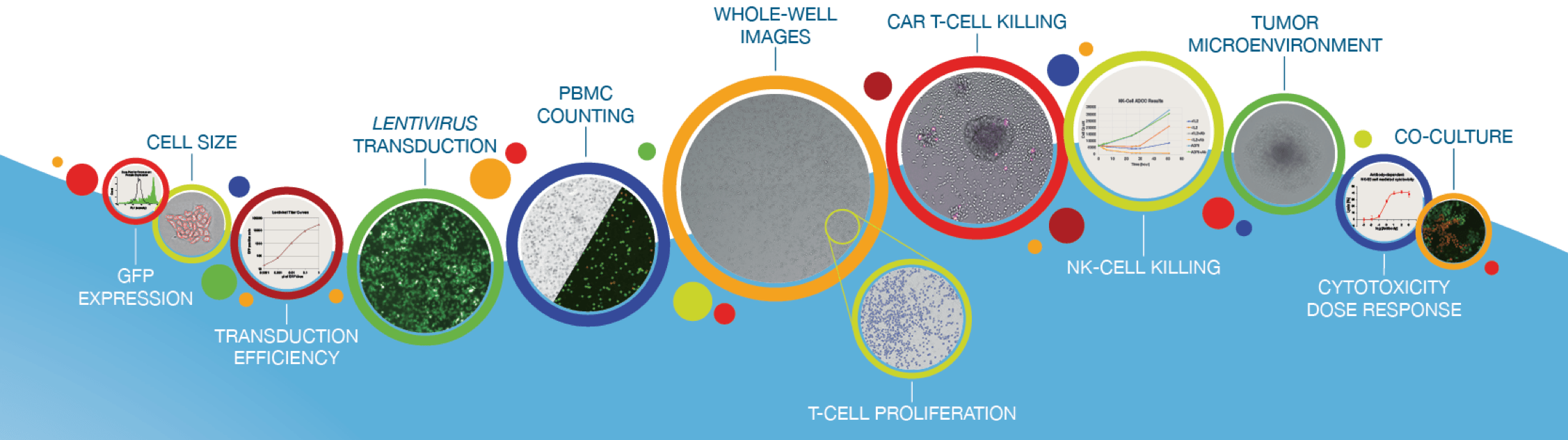
From Discovery to Manufacturing
Accurate Cell Counting Methods, Fit for Purpose, for CAR T Therapy
“Our Cellometer Auto 2000 has greatly improved the speed and accuracy of our cell counting and has allowed us to analyze our cell samples in a variety of ways. The AOPI feature has been integral in generating cell viable data for a few of our new projects. The customer service team is also amazing! They are very knowledgeable about their products and technology and are always available to help us troubleshoot.”
“The Cellometer Auto 2000 and the AO/PI Viastain gives us the ability to get an accurate count of our live splenocytes while excluding the red blood cells.”
“…definitely the standard for cell counting. Results are consistent time and again, the reliability is great when performing duplicate or triplicate counts…. I also enjoy the ability to establish several different settings based on the type of cell, and appreciate Nexcelom’s customer service!”
“The Cellometer Auto 2000 has greatly aided the development of our tissue dissociation assays. The unit allows fast quantification of viable white blood cells from a heterogeneous population following spleen, thymus, lymph node, etc. dissolution which can then quickly proceed to downstream flow-based analysis. The time saved by this method has been extremely valuable!”
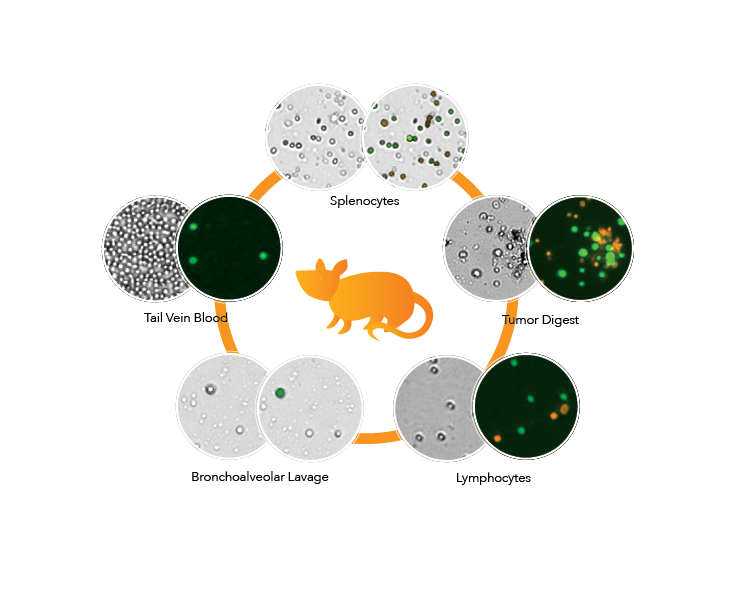
Cellometer provides accurate concentration and viability for cells that vary in morphology and heterogeneity.
Ezeh PC, et al. PLoS One. 2014; 9(4):e93920 | Xu H, et al. Toxicol Lett. 2016; 262:55-61
“We use the Cellometer Auto 2000 daily to count cells and assess viability of cells derived from a variety of sources. [We] perform a lot of cell line work, and need accurate counts and viability assessments before using the cells in downstream assays”
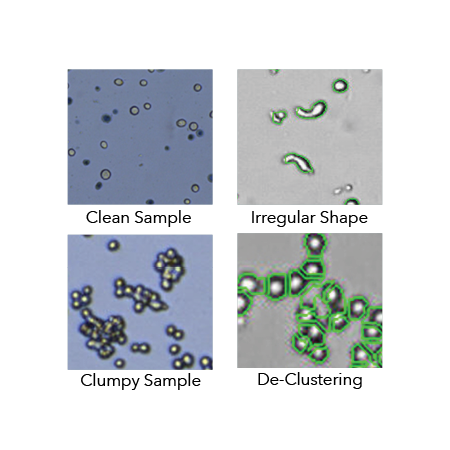
Cell counters need to handle NCI-60 cancer cell lines, of which 57% are clumpy, clustering, contain debris or have large variations in morphology.
Rothenberg SM, et al. Cancer Res. 2010; 70(6): 2158-64
Singh H, et al. PLoS One. 2013; 8(5): e64138 | Wang X, et al. Cancer Gene Ther 2015; 22(2):85-94
“The Cellometer Auto 2000 has made functional testing of T cells a much quicker and simpler process than before! When the cell concentration of each population in a 96 well plate must be determined, it is hard to imagine having to count the cells by hand and doing this in a timely manner. Great product!”
White KM, et al. ACS Infectious Diseases 2018; 4(2):146-157 | Zhang Z, et al. BMC Biotechnology 2018; 18(1):4
“…We routinely process PBMCs from both fresh whole blood and from frozen stock. The Cellometer [Auto2000] has made it much easier to get cell numbers and viability percentages for use in downstream applications …”
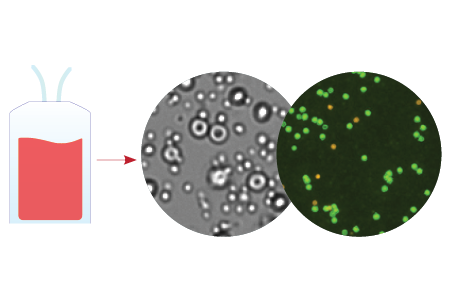
Manual / trypan blue method is not accurate for counting leukapheresis. RBCs are 3-12x the number of lymphocytes.
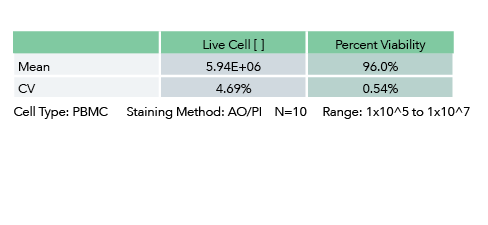
Proven Repeatability: 14 PBMC samples were tested using the Cellometer Auto 2000. The resulting in a CV of < 6%.
Burchiel SW, et al. Inhal Toxicol. 2016; 28(2):61-70

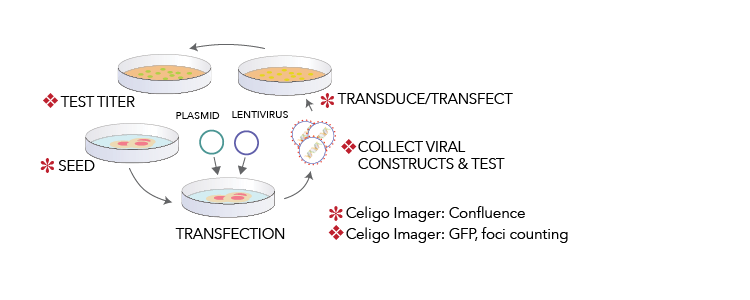
♠ Cellometer Cell Counting | ♦ Celigo Imaging Cytometer
Critical Quality Attributes
Safety
- Gram stain/sterility
- Mycoplasma
- Endotoxin level
- Copies of transgene insertion
- Replication Competent Retrovirus/Lentivirus ♦
Purity
- % CD3+ T cells ♠
- %CAR T cells ♠
- Residual tumor burden
- Residual beads
Identity
- % CAR T cells ♠
Potency
- In vitro Cytotoxic T Lymphocyte ♦
- Interferon-γ secretion ♠
Critical Process Parameters
- Cell growth and expansion ♠
- Selection of target cells
- Pre-stimulation conditions
- Transduction conditions ♦
Stability
- Shipping/Handling: Cryopreserved CAR T ♠
- Storage validation for Apheresis materials: Fresh/Frozen/Thawed CAR T samples ♠
- Vector
- Final product
≥70% cell viability is recommended for pre-clinical safety studies and clinical dose.
Cellometer K2 can measure cell viability from 0% viable to 100% viable cell population.
“I am currently using the Cellometer K2 in our lab, mostly to count T cells, PBMCs, and tumor cells. I use them for cell culture, and later the cells are used for further assays like ELISA and FACS. The instrument is very accurate, especially with AO/PI.”
Cellometer K2 Linearity Measurement
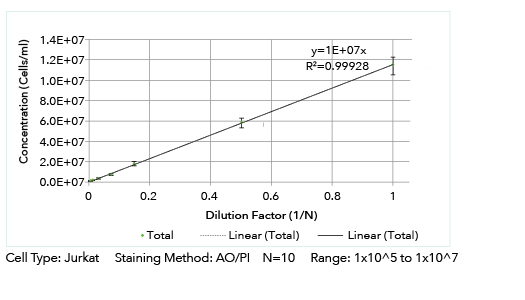
Accuracy translates from cell lines to primary samples.
Intermediate Precision: Instrument Repeatability and Validation
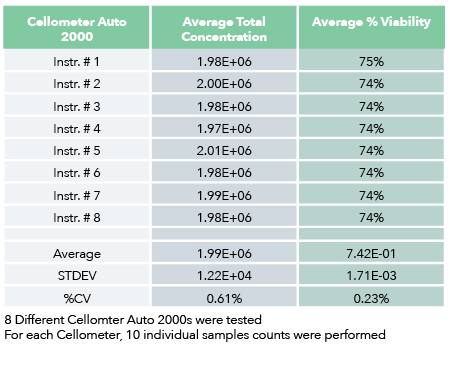
Cellometer cell counters have been facilitating CAR T manufacturing with accurate cell counting and analysis since 2010.
Precision: Repeatability of Cellometer K2