Engineering CHO cells using CRISPR technology
Nexcelom products help scientists quickly assess whether 1) CRISPR gene editing is successful and 2) if the engineered cells exhibit enhanced growth and viability without harvesting.
CRISPR is a relatively new technology that has the potential to revolutionize the field of biologics by targeting transgenes into specific sites to produce uniform cell populations with stable transgene expression. While CHO cells are a popular platform, the antibodies they produce are heavily fucosylated. This PTM decreases antibody-dependent cellular cytotoxicity and may inhibit therapeutic antibody function. Nonfucosylated antibody production is possible if the FUT8 gene in knocked out of protein production cell lines [Yamane-Ohnuki et al. 2004].
In the example below, scientists employed the optimized bioinformatics tool CRISPy to develop a chimeric single guide RNA (sgRNA) to disrupt the FUT8 gene [Ronda et al. 2014]. CHO cells were then cultured and transfected with Cas9 and sgRNA plasmids. After 2 days, the cultures were transfected with pmaxGFP to assess transfection efficiency using Nexcelom’s Celigo Image Cytometer.
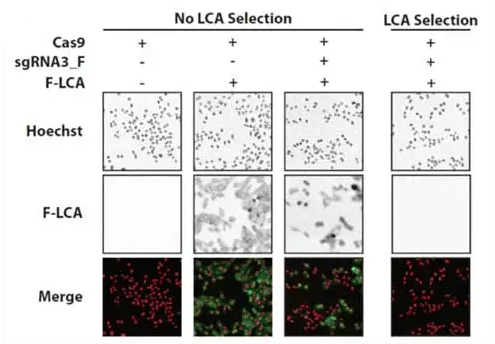
Phenotypic staining of FUT8 knockout CHO‐K1 cells by fluorescein‐labeled LCA (F‐LCA. CHO‐K1 cells were treated as described for panel A. On Day 13 of culture, cells were treated with Hoechst and with F‐LCA and fluorescence microscopy images were acquired. Hoechst and F‐LCA signals are shown as red and green, respectively, in the merged images and as grayscale in the individual images.
Five days after the initial transfection, the selection agent Lens culinaris agglutinin (LCA) was added. LCA binds plasma membrane proteins and ultimately causes cell death, but it is unable to do so in FUT8 knockout cells. Bright-field and fluorescent images were captured to confirm successful transfection. The bottom right panel shows that FUT8 was successfully knocked out in transfected cells subjected to LCA selection.
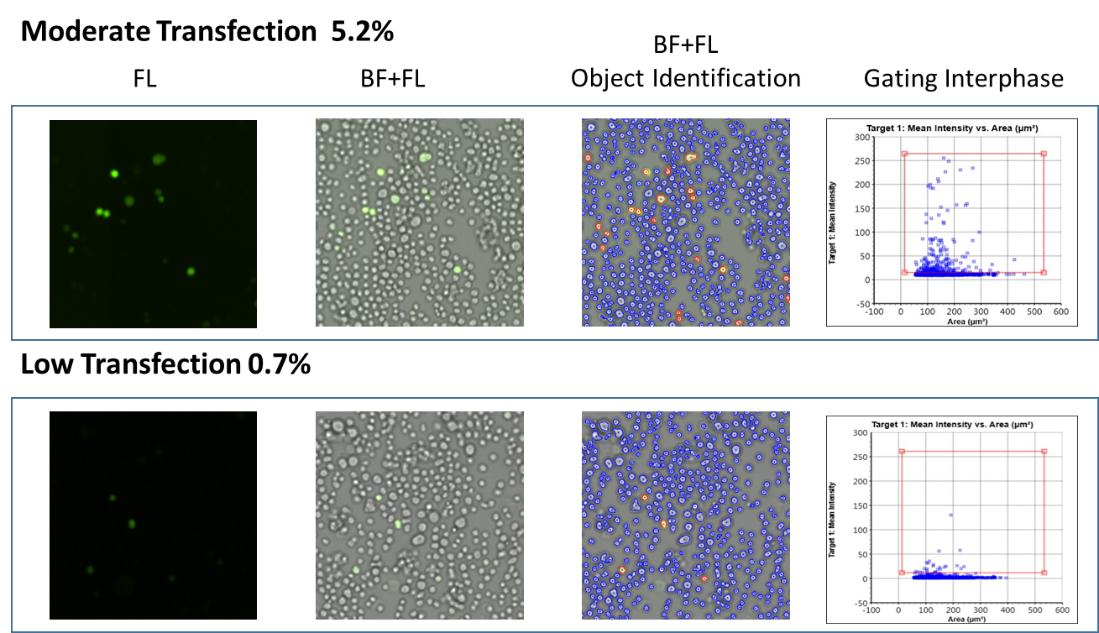
The Celigo Image Cytometer can quickly and easily monitor transfection efficiency to select and optimize cell lines. Lund et al. [2014] used Celigo to measure the transfection efficiency of CHO-S cells over multiple days. The merged brightfield and fluorescent images below show examples of moderate transfection (top) and low transfection (bottom).
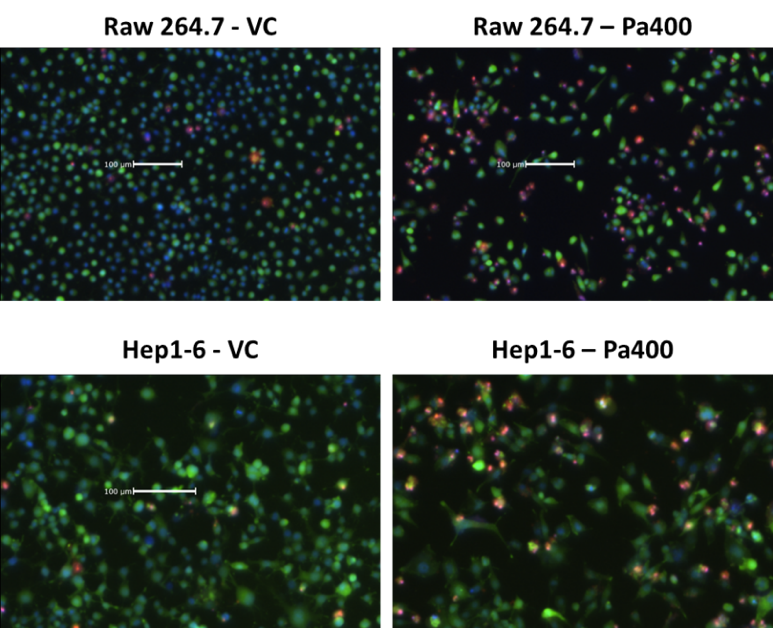
It is also important to measure cell viability to characterize the fitness of the cell line generated. In the example below, RAW 264.7 and Hep1-6 cells were stained with Hoechst (blue), calcein AM (green), and propidium iodide (PI, red) to determine the viability. The Celigo Image Cytometer was used to directly count the total cells (Hoechst), live cells (calcein AM), and dead cells (PI), and the results were used to calculate the viability percentages. Hansen et al. [2015] used the Celigo to directly measure the viability of CHO cells stained with Hoechst (blue) and PI (red). In this case, the cells were also expressing a split-GFP tag to measure the relative titer of therapeutic glycoprotein. Use of two fluorescent dyes to label total and dead cells did not limit Celigo’s ability to detect the GFP signal.
Engineering CHO cells using CRISPR technology
Learn more:
Engineering CHO cells using CRISPR technology
