Acute respiratory distress syndrome (ARDS) is characterized by pulmonary edema, often causing lung failure or death. ARDS occurs when the epithelial and endothelial cells within the lungs have a disrupted barrier function and fluids pass through and accumulate. This condition is the main cause of death in COVID-19 patients and there are no current drugs to cure or treat ARDS. Presently, treatment focuses on respiratory support with fluid management.
Discover potential drug candidates by studying barrier function
To discover potential drug candidates that can treat ARDS, in vitro high-throughput screening tools are vital. One way to determine endothelial barrier function in vitro is called the eXpress Permeability Test (XPerT) assay (Figure 1). High-affinity fluorescently labeled avidin are exposed to a confluent cell monolayer grown on a layer of biotinylated gelatin on the well of a plate. When the labeled avidin goes through the cell layer, it binds to the biotin and sticks to the bottom (Figure 1). Using an image cytometry like the Celigo allows fluorescent-labeled cells that pass through the cell barrier to be simultaneously detected and quantified. Modeling the endothelial cells in the lungs to study their barrier function, Dubrovskyi et. al. used human pulmonary artery endothelial cells (HPAECs) to form a monolayer of cells then quantified the barrier function by measuring green fluorescence on the Celigo.
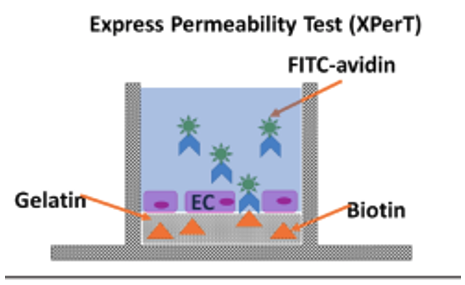
Figure 1: Using XPerT assay to measure endothelial barrier function. The lateral diffusion is measured by using biotinylated gelatin-coated cell culture plates. First the endothelial cells (EC) grow over the biotynilated gelatin coating. Next fluorescein-labeled biotin (FITC-avidin) is added to the cell media for a short period of time. Unbound FITC-avidin is washed out and cells are fixed by a formaldehyde solution. The permeability is visualized and quantified by FITC fluorescent area.
The Celigo is a whole-well image cytometer that automatically acquires images of microplates in brightfield and four fluorescent channels. In brightfield, the morphology of the cells in response to certain molecules changes can be detected. In addition, we could determine if the cell monolayer was disrupted to exclude it from the analysis. In the fluorescent green channel, the %confluence is measured to see how much FITC-avidin was able to pass through the cell barrier. Figure 2 shows example images of cells with high permeability versus a well containing cells with low permeability.
Use a whole-well image cytometer to automatically acquire images of microplates in brightfield and four fluorescent channels
The Celigo is a whole-well image cytometer that automatically acquires images of microplates in brightfield and four fluorescent channels. In brightfield, the morphology of the cells in response to certain molecules changes can be detected. In addition, we could determine if the cell monolayer was disrupted to exclude it from the analysis. In the fluorescent green channel, the %confluence is measured to see how much FITC-avidin was able to pass through the cell barrier. Figure 2 shows example images of cells with high permeability versus a well containing cells with low permeability.
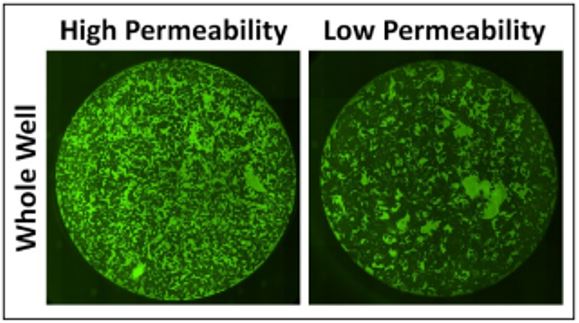
Figure 2. Celigo whole-well images of green fluorescent of FITC-avidin binding to an HPAEC monolayer on a gelatin-coated surface of a 96-well plate. The images compare FITC-avidin with cells with high-permeability HPAECs versus low-permeability HPAECs.
To test if the Celigo could measure the changes in permeability, known signaling factors were used to affect cell permeability. In ARDS, the endothelial paracellular pathways are known to be misregulated. Adherens-junctions and focal adherens junctions are known to mediate cell-cell connections and are linked to the actin cytoskeleton. Signaling molecules such as Thrombin, tumor necrosis factor-alpha (TNF-a), and lipopolysaccharide (LPS) are known to affect barrier function. Here time-course assays were performed by treating the cells with these factors to see how the Celigo can detect the changes in cell permeability (Figure 3).
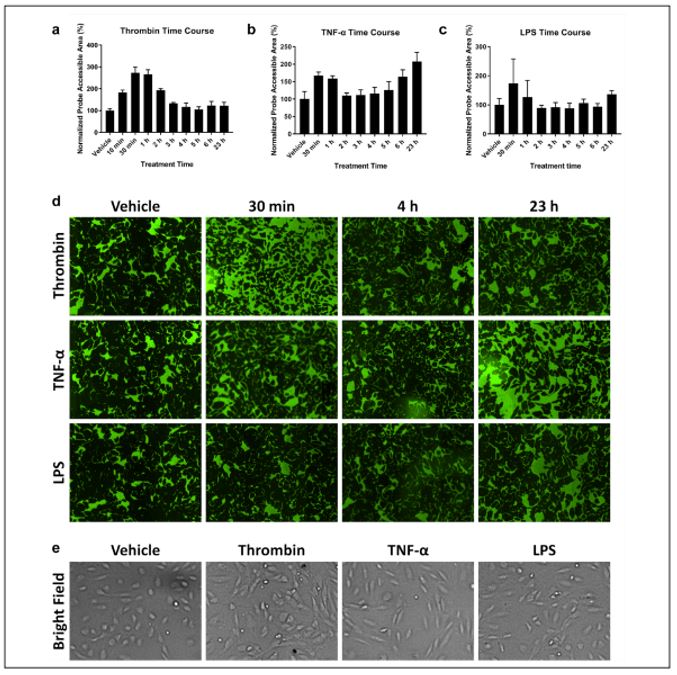
Figure 3. Cell permeability was compared using a time-course XPerT assay performed on cells treated with with (a) thrombin, (b) TNF-a, and (c) LPS for 23 hours. (d) Time-dependent green fluorescent images show changes in cell permeability due to the soluble factors indictated by the fluorescent area. Thrombin showed a large fluorescent area between 0.5 and 2 h, and both TNF-a and LPS showed a larger area at 23 h. (e) Brightfield images show morphological changes due to soluble factors (vehicle, round; thrombin, smaller; TNF-a, elgonated; LPS, both morphologies).
This study indicated several advantages to using the Celigo Image cytometer over other current methods. We were able to not only measure the confluence of the fluorescent channel but image the monolayer to inform the interpretation of the data. Any disruption of the monolayer or morphology changes were able to be detected and incorporated into the analysis. We were also able to improve the signal-to-noise ratio due to the ability to threshold fluorescent intensity in permeable areas. This approach is adaptable for even more high throughput options and can be scaled to 384 well plates to test even more molecules. Overall, the Celigo is a vital tool for improving in vitro modeling and can be utilized to discover potential drug candidates to help treat ARDS.
Implementing the Celigo Image Cytometer opens the avenue for a more in-depth analysis in a high-throughput and potentially automated fashion. Learn more about cellular models and the direct cell counting assays on our Celigo Image Cytometer Applications page or contact us directly to book a seminar or live demonstration today!
Dubrovskyi, Oleksii, et al. “Development of an Image-Based HCS-Compatible Method for Endothelial Barrier Function Assessment.” SLAS DISCOVERY: Advancing the Science of Drug Discovery, vol. 26, no. 9, Oct. 2021, pp. 1079–1090, doi:10.1177/24725552211030900.






Leave A Comment