Antibody-directed and drug therapies have been at the forefront of immuno-oncology research in the past 15 years. Since the FDA introduced the Breakthrough Therapy Designation in 2016 for novel promising drug therapies, the number of applicants and approved candidates has increased dramatically.
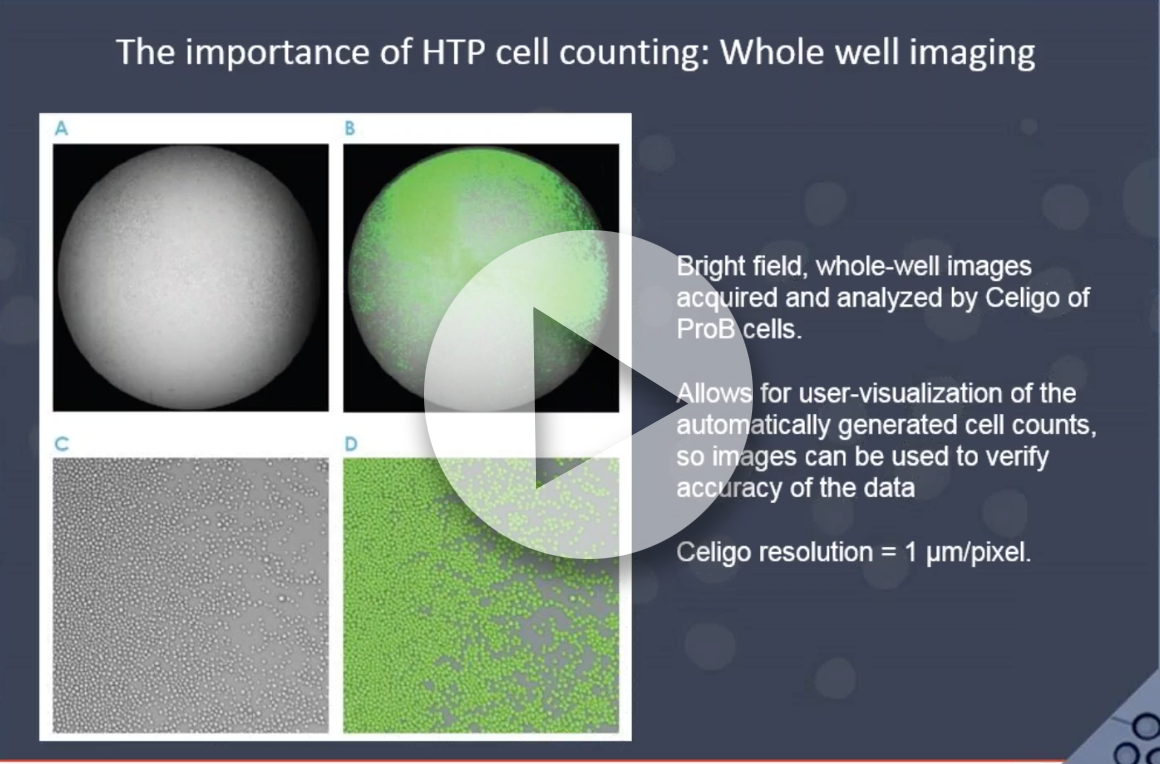
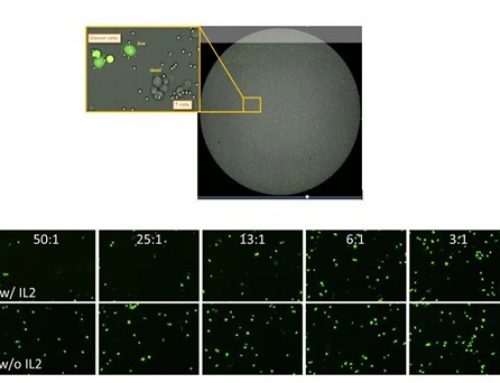
As more and more researchers are looking at drug effects in cancer cells and moving forward in drug discovery, they face many challenges in their day to do work. Performing cell cytotoxicity assays for multiple cell lines with single and combination drug therapies is standard practice in immuno-oncology. While many researchers use end-point assays such as MTT, XTT, WST, or CellTiter-Glo, others prefer the manual counting using Trypan Blue. While these methods may be popular, it does not mean they are without their limitations. By directly imaging and counting every cell in a well over a course of a drug treatment, the Celigo can perform the cytotoxicity assays in a label-free format.
We recently presented an informational webinar on this very topic. If you’re intrigued to learn what advantages and disadvantages we identified for these methods, we encourage you to watch the webinar. You can access it here.
Once you’ve had a chance to watch it, we’d love to hear your thoughts, opinions, and any questions you may have.




Leave A Comment