Introduction
Preclinical models to evaluate therapeutic efficacy in the area of oncology have proven to be a challenge. The standard of preclinical assessment as always been immunosuppressed mice, but that model has not reliably predicted clinical efficacy [1]. Immunocompetent genetically engineered mice with tumor allograft models seem more promising, and yet reporter tags such as GFP and luciferase are targeted by competent immune systems, which may interfere with tumor growth and the response to therapeutics. Here, a collaborative group of researchers has created a reporter-tolerized genetically engineered mouse model, dubbed “Glowing Head” (GH), by targeting a luciferase-GFP reporter into the anterior pituitary gland [2]. These mice have been programmed to be immunologically unresponsive to this reporter construct, thereby making them ideal hosts for labeled syngeneic tumors in order to study preclinical anti-tumor therapies.
Materials and Methods
Wild-type (WT) and GH mice carrying subcutaneous Lewis Lung Carcinoma (LLC) tumors received vehicle or paclitaxel (Tx) at doses mimicking human doses.
Single-cell suspensions of harvested mouse spleens were incubated with rat anti-mouse CD4 or CD8 antibodies with an isotype control. An Alexa Fluor 488-conjugated goat anti-rat secondary antibody was applied and cells were analyzed on the Cellometer Vision CBA for FITC expression to determine the ratio of the CD8+ to CD4+ subpopulation (CD8/CD4) in WT and GH mice. All data were quantitated with FCS Express software.
*Note: For more detailed Materials and Methods and a complete account of the entire study, please refer to the original manuscript [http://www.ncbi.nlm.nih.gov/pubmed/25369133]
Results
- CD8/CD4 ratio of splenocytes from WT mice treated with Tx were significantly increased from vehicle-treated WT levels, but the same trend was not seen in the Tx-treated versus vehicle-treated GH animals. (Figure 1A)
- There was strong correlation between CD8/CD4 ratios and spleen weight in all treatment groups. (Figure 1B) (R2=0.071)
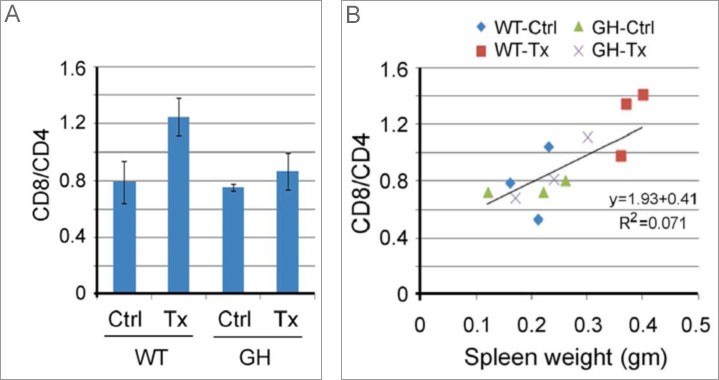
Figure 1A and B. Immunogenicity of reporter construct alters the chemoresponsiveness of tumors in WT mice compared to GH mice
Conclusions
- There is a keen need for immunocompetent mouse models to assess preclinical anti-tumor therapies as the data from immunosuppressed mouse models have not been shown to translate into the clinic.
- Xenobiotic reporters are problematic for immunocompetent mice, but the GH mice were successfully tolerant of the reporter construct utilized here and address many of the limitations of other immunocompetent mouse models.
- This opens the door for virtually any reporter-tolerized mouse model.
- The CD8/CD4 ratio is an indicator of immune system function. The WT animals demonstrated a significant increase in the ratio between treated and untreated WT animals, but that increase was not seen between treated and untreated GH animals.
References
- Administration USFaD, Innovation or stagnation: challenge and opportunity on the critical path to new medical products, 2004.
- Day, C. and G. Merlino, Immunocompetent mouse model for tracking cancer progression, Health and Human Services, 2011: Federal Register.


Leave A Comment