Introduction
A diverse family of cytokines, Type I interferons (IFNs), is a group of proteins responsible for antiviral, antiproliferative, and immunomodulatory functions [1, 2]. Treating cells with Type I IFNs induces autophagy, a cellular recycling process that is considered a key cell survival strategy [3]. It is well known that Type I IFNs work through the JAK-STAT pathway, but recent evidence suggests that the MAP kinase pathway can affect the expression of IFN-regulated genes [4, 5]. Researchers demonstrated that IFN-induced autophagy affects cell cycle as well as cellular proliferation in a variety of cell lines. Because autophagy plays a major role in cancer, it is important to understand how IFNs may regulate it.
Materials and Methods
Cell culture
Seven different cell lines grown in the presence of absence of autophagy-inducer IFNA2c were used to analyze IFN-induced autophagy: Daudi, T98G, MDA-MB-231, HeLaS3, BJAB, A549, and U937. All lines were grown in RPMI media.
Cell growth and viability
Cell counts and viability measurements with and without exposure to IFNA2c were taken using Trypan Blue and Cellometer Vision.
Cell cycle analysis
A Cellometer image-based cytometer and Cell Cycle PI kit were used to investigate the cell cycle effects of Type I INFs on the cells in culture.
Autophagy analysis
A Cellometer image-based cytometer and Cyto-ID autophagy detection kit were used to analyze the effects of Type I INFs on cellular autophagy. Autophagy Activity Factor (AAF) reports the differences in amounts of green dye-autophagosomes accumulated in the cells in the presence and absence of an autophagy inducer (IFNA2c).
*Note: For more detailed Materials and Methods and a complete account of the entire study, please refer to the original manuscript [http://www.ncbi.nlm.nih.gov/pubmed/23419269]
Results
- After 48 hours with or without IFNA2c treatment:
- there was a significant increase in growth inhibition in all cell lines but one, which correlated with an increase in molecular autophagy markers;
- Autophagy, as measured by AAF, showed large increase in autophagic activities in some cell lines, while others showed only a small increase
- The number of Daudi cells in G1 phase of the cell cycle increased.
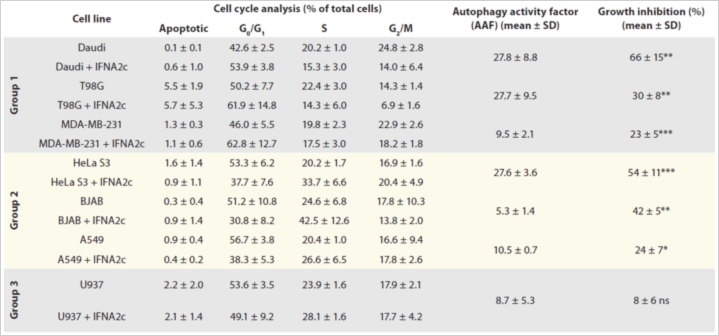
Table 1. IFNA2c’s effect on autophagy, cell cycle, and proliferation. Phase populations were reported as Apoptotic, G0/G1-, s- or G2/M-phase. Autophagy Activity Factor is also reported. Data shown are averages of three individual experiments, ± SD of experimental replicates. *p < 0.05 (significant); **p < 0.01 (very significant); ***p < 0.001 (extremely significant); ns: p > 0.05 (not significant).
Conclusions
- Here, researchers demonstrated novel ways by which Type I IFNs direct autophagy.
- In all seven cell lines tested, IFNA2C significantly increased molecular autophagy markers and the percentage of growth inhibition and demonstrated alterations in the cell cycle.
- Future studies are required to assess the detailed mechanisms of Type I IFN-mediated autophagy in various cell types.
- Pharmacological manipulation of Type I IFN-induced autophagy could provide novel therapeutic opportunities.
References
- Bekisz, J., et al., Antiproliferative Properties of Type I and Type II Interferon. Pharmaceuticals (Basel), 2010. 3(4): p. 994-1015.
- Bekisz, J., et al., Human interferons alpha, beta and omega. Growth Factors, 2004. 22(4): p. 243-51.
- Kroemer, G. and B. Levine, Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol, 2008. 9(12): p. 1004-10.
- Kessler, D.S., D.E. Levy, and J.E. Darnell, Jr., Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci U S A, 1988. 85(22): p. 8521-5.
- Platanias, L.C., The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther, 2003. 98(2): p. 129-42.


Leave A Comment