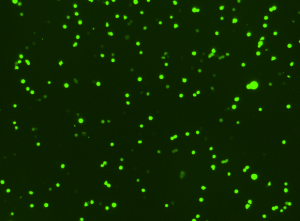
GFP and Determination of Transfection Efficiency
Green Fluorescent Protein (GFP) is a 26.9 kDa protein that fluoresces bright green when exposed to blue or ultraviolet light. GFP was first identified as a protein and extracted from the Aequorea victoria jellyfish in 1962 by Osamu Shimomura, et al. The GFP protein was first cloned in 1992 and it was soon confirmed that GFP protein expressed in other organisms generates fluorescence. An area within a cell or tissue is briefly illuminated, causing the GFP protein to fluoresce, allowing GFP-tagged proteins to be identified. In 2008, Osamu Shimomura, Marty Chalfie, and Roger Tsien received the Chemistry Nobel Prize “for the discovery and development of the green fluorescent protein.” In recent years, various mutations have yielded forms of GFP offering improved brightness, a modified excitation and emission spectra, improved 37°C folding, reduced aggregation at high concentrations, and improved diffusibility inside cells. Today, GFPs are used in an increasing variety of cell-based assays to measure intra-cellular events. GFP genes are co-transfected with genes of interest to measure transfection efficiency, GFP fusion tags are used to identify the localization and fate of normal host proteins, and specifically-engineered GFPs are used as indicators of phosphorylation.
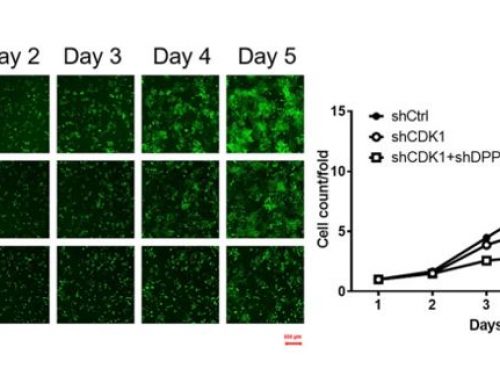
With the Cellometer Vision Image Cytometry Systems, researchers can easily quantify the percentage of GFP-positive cells within a population, allowing for fast, accurate determination of transfection efficiency. Researchers can overlay histograms for control and treated samples for advanced gating of GFP- and GFP+ cell populations.
1Tsien, Roger Y., (1998). The Green Fluorescent Protein, Annu. Rev. Biochem. 67:509-44.
2http://www.conncoll.edu/ccacad/zimmer/GFP-ww/timeline.html,accessed September 25, 2012.




Leave A Comment