Increase Accuracy and Accelerate Vaccine and Antiviral Compound Assessment
There are currently no approved antiviral drugs or recommended vaccines to prevent and or treat Feline coronavirus (FCoV) infections. Researchers at Colorado State University decided a faster and more sensitive method was needed over traditional plaque reduction neutralization tests (PRNT). As described here, scientists developed a novel high-throughput viral microneutralization assay for faster and more accessible quantification of virus-infected cells, testing of antiviral drugs, and investigation of pathogenesis using a plate-based image cytometer.
“The Celigo Image Cytometer reduces assay time while providing richer, quantitative readouts to accelerate assessment of vaccine and antiviral compound candidates. Importantly, the optimization strategy can be employed to adapt other cell types to the assay format or to create entirely new virus-host cell combinations.”
Simultaneously Perform Whole-well and Whole-plate Imaging and Analyses
In this work, the Celigo Image Cytometer was used with the brightfield as well as green and blue fluorescence imaging channels to detect and count adherent epithelial cells derived from feline kidney cells (CRFK) that were infected by FCoV. The infected cells were first fluorescently stained with Alexa Fluor 488 (AF488)-labeling and DAPI. After performing automatic focus of the cells in each well, the Celigo was used to simultaneously performed whole-well and whole-plate imaging and analyses to generate percentages of green fluorescent infected cells.
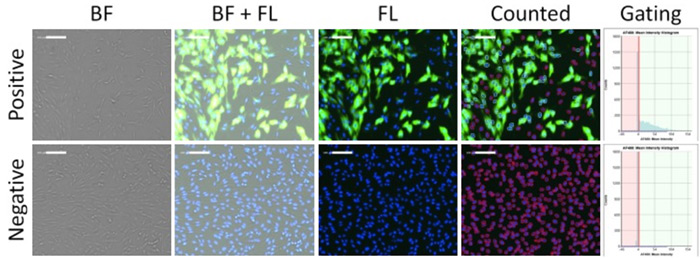
Figure 1. Brightfield and fluorescent images, and fluorescence intensity gating of positive and negative infection of CRFK cells using optimized scanning and analysis settings from image cytometry
First, the software identified the total number of DAPI-positive host cells using the blue channel. Next, the AF488 fluorescent intensities were measured to determine what cells were positive for the infection to determine the infection percentages. Multiple parameters were investigated to optimize the assay including cell seeding density, plate surface coating, virus concentration, incubation time, wash buffers, and fluorescent labeling.
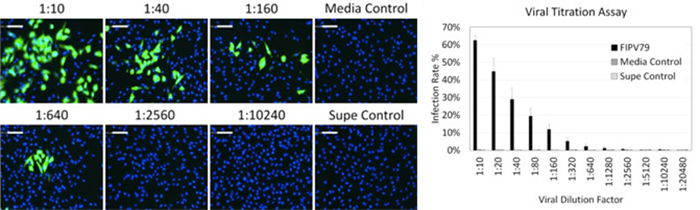
Figure 2 Viral concentration optimization results. (left) Viral concentration-dependent fluorescent images and (right) infection rates measurement showing the reduction of AF488-positive infected CRFK cells as viral concentration decreased. Both media and supernatant control did not show viral infection.
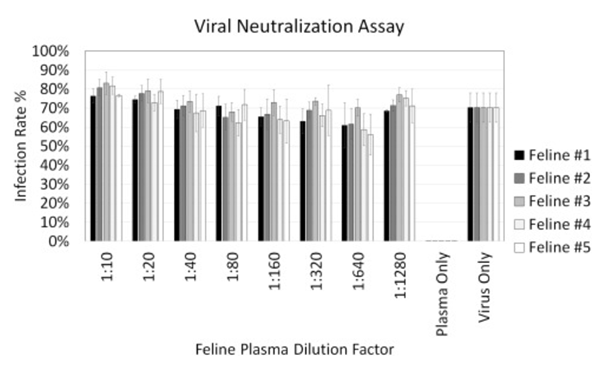
Figure 3. Viral neutralization results showing dose-dependent infection rates measurements for five feline plasma samples with a titration from 1:10 to 1:1280. The virus and plasma only conditions showed high and low infection rates as expected. No noticeable neutralization effects were observed.
The optimized detection method was then employed for performing a high-throughput microneutralization screening of potential FCoV antibodies using five collected feline sera against the Feline coronavirus strain FCoV-WSU-79–1146. The high-throughput neutralization assay was conducted twice. Due to the lack of cross-neutralization between FCoV type I and II viruses from the feline sera, no viable candidates were identified.
Perform a Microneutralization Assay in Approximately 15 Minutes per 96-well Plate
Traditional methods for screening vaccine and therapeutic candidates as well as assessing immune responses are typically time-consuming, low-throughput, and can require several days. Lack of digital autosave makes recordkeeping problematic and repeatability is also a concern with multiple users producing varying results. The Celigo saves time by simultaneously imaging and analyzing data with digital autosave that makes recordkeeping effortless. Additionally, preset features save time, resources, and prevent extended user training or operator variability issues. “The image cytometer performed the neutralization assay at approximately 15 min per 96-well plate for simultaneously acquiring and analyzing whole-well images in brightfield and fluorescence, equivalent to ~9 s per sample.”
High-throughput viral microneutralization method for feline coronavirus using image cytometry
First Author: Morgan Pearson, Department of Microbiology, Immunology, and Pathology
First Author Institution: Colorado State University, Fort Collins, CO, 80523, United States
Journal: Journal of Virological Methods






Leave A Comment