Plaque assays remain one of the most commonly used methods for the direct quantification of infectious virions and antiviral substances through the counting of discrete plaques in cell cultures. This technique was first used to calculate the titers of bacteriophage stocks and it has since been used for reliable determination of the titers of a number of different viruses1.
Plaque Assays: The Traditional Method
During a plaque assay, a confluent monolayer of host cells is infected with a lytic virus of an unknown concentration, which has been serially diluted to a countable range, typically between 5-100 virions per well. Depending on the viral growth kinetics and host cell used, a visible open plaque (area without host cells, lysed by the virus) will typically form within 2-14 days. After fixing and staining the infected cellular monolayer, plaques are counted so that titer viral stock samples in terms of plaque-forming units (pfu).
Improved Plaque Assay Method for Earlier Detection
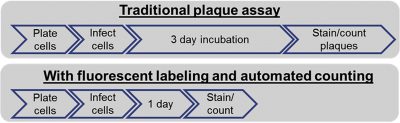
Sometimes, the target virus does not induce lysis of the host cells, thus would require either colorimetric or fluorescent labeling of the infected host cells to reveal viral plaques. In a recent article published in Molecular Therapy: Methods & Clinical Development2 published by Masci et al. the authors described how typical plaque assay protocols performed with horseradish peroxidase-conjugated antibody and substrate subsequently counted by eyes are cumbersome, time-consuming, and prone to operator error. Instead, they proposed a fluorescent method using fluorescently-labeled antibodies and the use of the Celigo image cytometer to reduce the amount of time required for the assay to incubate prior to automated image acquisition. As shown in the diagram in Figure 1, the fluorescent method allowed the reduction of the assay length from six to seven days down to only four days.
Figure 1 Comparison of traditional plaque assay workflow with image cytometry workflow
Reliability of Fluorescence Plaque Assay Method
The authors also demonstrated the reliability of the fluorescent method by comparing it to the traditional manual counting method. An excellent correlation was observed between the two methods. More importantly, they show how using the fluorescent method allows the quantification of the number of plaques after 24h of incubation while it previously took up to 72h for the traditional assay to develop. Just as important, they show that the number of fluorescent plaques detected at any time after 24h remains consistent bringing additional robustness to the assay (Figure 2).
Figure 2 Plaque counting and visualization using the fluorescent staining versus HRP Staining
Conclusion
The authors concluded that “by utilizing automated counting and a computer algorithm for counting plaques, analyst subjectivity is removed. Using the Celigo imager also significantly reduces plaque counting time and improves data consistency”.
1https://www.creative-biogene.com/support/Viral-Titering-Plaque-Assay-Protocol.html







Leave A Comment