Introduction
The B-cell lymphoma-2 (BCL-2) family of proteins includes many key regulators of cell survival, cell death, and apoptosis. The deregulation of certain BCL-2 proteins is one of the first steps towards tumorigenesis and subsequent therapeutic resistance [1-4]. Targeting these BCL-2 family members is therefore an important area of oncology research [2, 4, 5]. Here, a collaboration of researchers has explored the targeting of these BCL-2 proteins with a BCL-2 binding peptide known as NuBCP-9 (FSRSLHSLL), which induces a conformational change in BCL-2 that alters the protein’s function from cell protection to cell killing. Researchers paired NuBCP-9 with iron oxide-based nanoparticles (supramagnetic iron oxide nanoparticles; SPIONs) enhanced through bio-cleavable bonds using other alterations to improve limitations of other previously studied delivery methods. When exposed to MCF-7 and HepG2 cells, these NuBCP-9-SPIONs induced significant apoptosis of those cells in culture.
Materials and Methods
Cell culture
MCF-7 (hormone-dependent breast carcinoma) and HepG2 (heptocarcinoma) cells were maintained in DMEM medium supplemented with 10% fetal bovine serum at 37°C.
NuBCP-9 application
To determine the effect of encapsulated NuBCP-9 on cell proliferation, cells treated with NuBCP-9, NuBCP-9-R8, SPIONs and NuBCP-9-SPIONs using multiple concentrations of these test materials. Cells were seeded in 96-well culture plates and incubated followed by treatment with different NuBCP-9 formulations.
Assessment of apoptosis
Cells were stained using the Annexin V-Alexa Fluor 488/PI Apoptosis Assay Kit Quantification of apoptosis/necrosis was performed using Cellometer Vision.
*Note: For more detailed Materials and Methods and a complete account of the entire study, please refer to the original manuscript [http://www.ncbi.nlm.nih.gov/pubmed/25340469]
Results and Discussion
Nanosystem preparation
- Synthesized nanoparticles were characterized by different techniques (TEM, XRD, and SQUID). The size of the nanoparticles ranges from 7 to 10 nm and are superpara-magnetic.
- These nanoparticles are highly dispersible as shown in our previous studies [6].
Cell culture apoptosis studies
- To assess the effects of NuBCP-9- SPIONs on the apoptotic response, MCF-7 cells were treated with NuBCP-9-SPIONs and monitored for externalization of phosphatidylserine (PS) at the cell membrane.
- Quantitation of annexin V and PI staining using Cellometer Vision confirmed that NuBCP-9-SPIONs are as effective as NuBCP-9-R8 (cell penetrating peptide-linked NuBCP-9) in inducing apoptosis of MCF-7 cells at 48 h (Fig. 1). As additional control, NuBCP-9 devoid of r8 had little, if any, effect on MCF-7 cell apoptosis (Fig. 1).
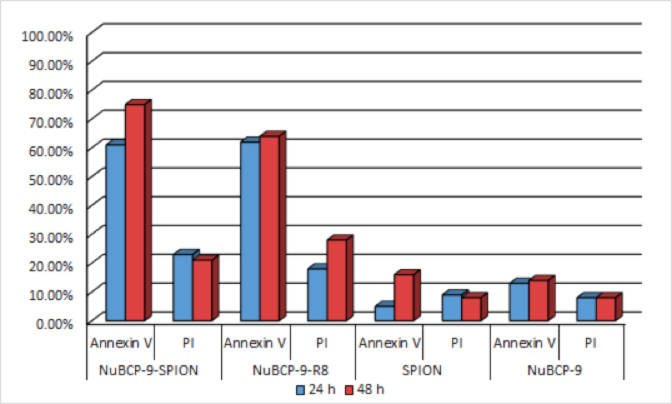
Figure 1. Measurement of apoptosis in BCL-2 overexpressing cells. Quantification of apoptosis/necrosis in MCF-7 cells by Annexin V/PI staining and treatment with NuBCP-9- SPIONs and NuBCP-9-R8 for 24 h (blue) and 48 h (red), using Cellometer Vision. Annexin V+/PI− is an indicator of early apoptosis and Annexin V+/PI+ late apoptosis/early necrosis.
Conclusions
- These findings describe a novel iron oxide nanoparticle-based approach for the delivery of L-amino acid peptides without a cell-penetrating domain that:
- Can be delivered through nanocrystals,
- Can be used for diagnosis and treatment,
- Induce effective anticancer activity in vitro.
References
- Ashkenazi, A., Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov, 2008. 7(12): p. 1001-12.
- Borden, E.C., H. Kluger, and J. Crowley, Apoptosis: a clinical perspective. Nat Rev Drug Discov, 2008. 7(12): p. 959.
- Cotter, T.G., Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer, 2009. 9(7): p. 501-7.
- Yip, K.W. and J.C. Reed, Bcl-2 family proteins and cancer. Oncogene, 2008. 27(50): p. 6398-406.
- Szakacs, G., et al., Targeting multidrug resistance in cancer. Nat Rev Drug Discov, 2006. 5(3): p. 219-34.
- Kumar, M., et al., Cellular interaction of folic acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int J Nanomedicine, 2012. 7: p. 3503-16.


Leave A Comment