Patients with cancers such as multiple myeloma, leukemia, lymphoma, and other metastatic cancers have tumor cells with unique immunological targets that when exploited, can lead to the complete destruction of the mass and a lasting remission from disease. Clinicians attack those targets using cell therapy, also known as “targeted immunotherapy”. Cell therapy is the programming of a patient’s own immune cells to target that patient’s tumor cells for destruction. One application of cell therapy, Adoptive Cell Transfer (ACT), employs select methods of genetic manipulation and propagation of those immune cells ex vivo so that the newly programmed cells can be reintroduced into the patient to specifically eliminate the tumor. (Figure 1.)
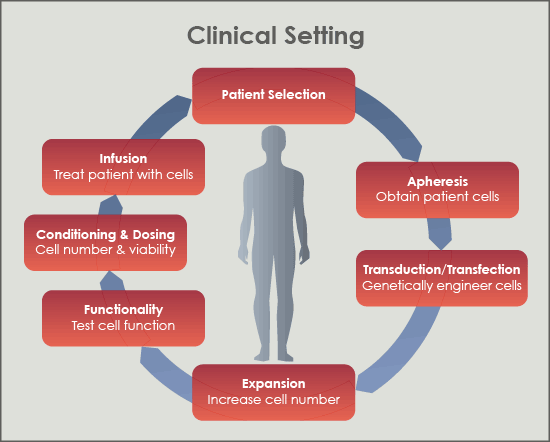
Figure 1. Overview of Adoptive Cell Transfer (ACT) process where immune cells are removed from the patient, genetically manipulated, propagated, tested for function, analyzed for number and viability, and reintroduced into the patient to target tumor cells.
At the Department of Stem Cell Transplantation & Cellular Therapy at MD Anderson Cancer Center, researchers employ Nexcelom’s Cellometer Vision CBA and K2 using Adoptive Cell Transfer to treat patients with multiple myeloma, leukemia, and lymphoma. Cell therapy research is challenging because there is a great diversity of cell populations and sizes that must be counted. Samples must be pure, the technique must be sensitive and specific for the desired cell populations, and any debris must be excluded from the calculations. With Cellometer Vision and K2, the MD Anderson team was able to overcome these challenges, and utilize antigen presenting cells (APCs) to specifically stimulate a patient’s T cells in the process shown in Figure 2A and B.
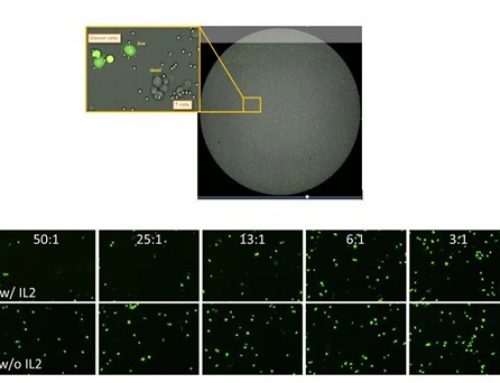
As can be seen below, Cellometer is integral to multiple steps throughout the process: ensuring proper concentration and culturing conditions of both the APCs and the T cells, as well as the functional analysis of the T cells before and after stimulation by the APCs. The latter is achieved by the addition of a tracer dye to the cancer cells. As the T cells successfully lyse the cancer cells, the dye disappears, and this is measured by Cellometer. Once the T cells are capable of lysing the cancer cells, the T cells are counted and frozen down before thawing and the proper concentration adjustments are made for them to be reintroduced into the patient. See Figure 3 for representative Cellometer images.
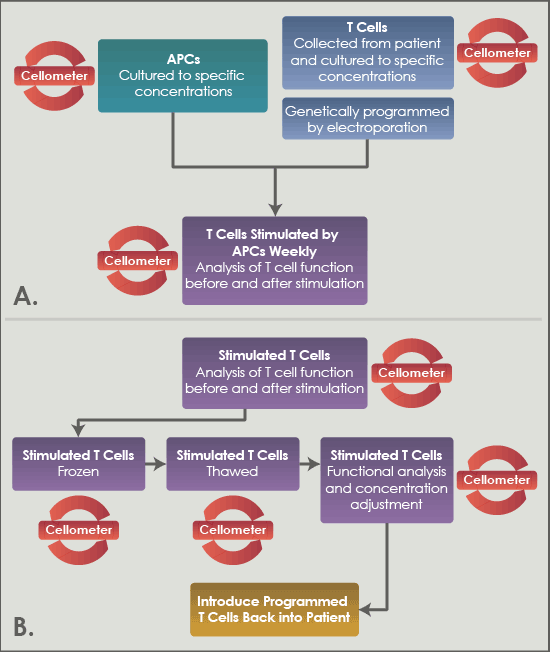
Figure 2A and B. MD Anderson’s ACT procedure with the Cellometer for producing programmed T cells to reintroduce into the patient. Everywhere the yellow circular arrows are shown, Cellometer is used to reach the next step.
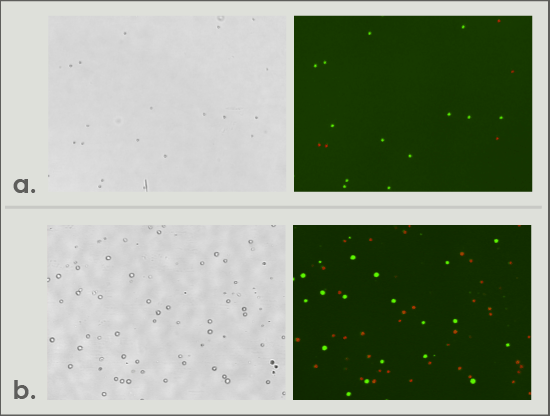
Figure 3. (a) T cells stained with AOPI for concentration and viability measurement using Cellometer K2. (b) APCs stained with AOPI for concentration and viability measurement using Cellometer Vision.
Cellometer is capable of using over 15 different cell types for cell therapy research, both primary and cell lines, both human and murine. Cellometer achieves accurate, consistent results using Trypan Blue alone or in combination with fluorescent markers with only 20μL of sample. (See Figure 4 for a full list of cell types and viability methods used with Cellometer.)
[table width=”550″ colwidth=”275|275″ colalign=”left|left”]
Cell Type, Viability Measurement
Hybridoma cells, TB
293T cells, TB
T-cells for expansion, TB
Tumor digest, AOPI
Isolated B-cells, AOPI or TB
Splenocytes – Fresh, AOPI
Splenocytes after RBC lysis, AOPI or TB
PBMC – isolated, TB
PBMC – fresh, AOPI
Feeder cells (K562 cells), TB
[/table]
Figure 4. Cell types used in cell therapy and analyzed on Cellometer using either trypan blue (TB) or the fluorescent mixture of acridine orange (AO) and propidium iodide (PI) for analysis.
References
1.Restifo NP, et al. Adoptive immunotherapy for cancer harnessing the T cell response. Nature Reviews Immunology.Vol.12, April, 2012.
2.High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. Zhao Y, et al. Journal of Immunology. Vol. 179 (9), November 1, 2007
3.Reprogramming CD19-specific T cells with IL-21 signaling can improve adoptive immunotherapy of B-lineage malignancies. Singh H, et al. Cancer Research. Vol. 71(10), May 2011
For more information:



Leave A Comment