Using old tools to create new ones
Repurposing immune cells to develop new therapies
For the development of new drugs and the advancement of new cell therapies, pharmaceutical companies have recurred to cytotoxicity assays as a quick way to assess the viability of cell lines in response to an external stimulus. This stimulus can be in the form of small molecules, synthetic drugs, or other cell types like CAR-T and NK-cells. Cytotoxicity assays provide critical biological information about cell responses to external stimuli and the potency of such drugs to elicit cell death. In the process, these assays, help in the development of safe and effective treatments by identifying compounds with the highest probability of success.
Advantageous factors for method considerations
When designing a cytotoxicity assay, it is essential to consider various factors including the external stimulus, the throughput of the analysis, and how the killing assay is prepared and performed. For instance, cancer immunotherapies utilize chimeric antigenic receptors (CAR) T cells and primary natural killing (NK) cells. Each cell type has its advantages at the time of inducing cell death. CAR-T cells require a specific antigen to direct them to the target cell and cause killing. While NK cells, whether primary or controlled by an antigen (CAR), have spontaneous cytotoxic activity.
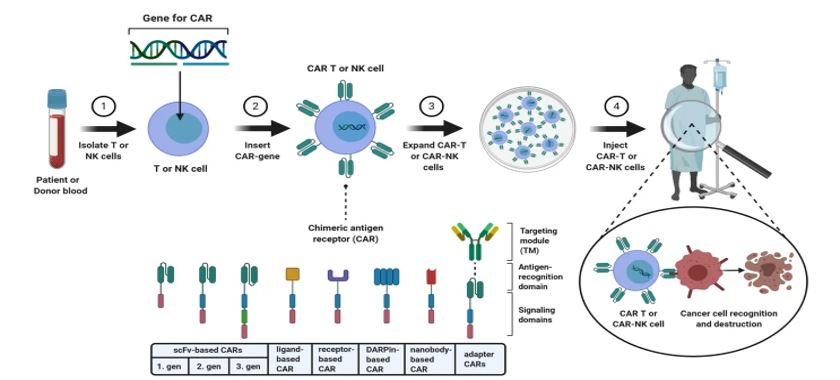
Figure 1: CAR-T and CAR-NK cell therapy. Albinger, N. etal. 2021
Whether the cytotoxicity assay uses CAR-T cells or NK cells, live and dead cells have to be identified to determine the percentage of cell death. Many studies use flow cytometry and nuclear dyes (AO/PI, DAPI/PI, 7-AAD, DRAQ7, etc..) to measure the viability of cells in a microfluidic format. This method is highly effective, sensitive, yet time-consuming. In many situations, biologically relevant information from the cell culture environment is lost due to cell monolayer disruption. On the other hand, image cytometry is an alternative technique to monitor cytotoxicity over time that consists of taking images and analyzing cell populations. In this format, biologically relevant information originating from cell-cell contact is retained, giving the user relevant information that might be lost upon cell monolayer disruption.
Key considerations when preparing cytotoxicity assays
This article details all the steps required to activate NK cells in vivo using poly(I:C) in preparation for the ex vivo cytotoxicity assay. This protocol aims to monitor short-term cell death mediated by Granzyme B produced by NK cells. In summary, 48 hours before killing assay, the target cell line and IL-15 stock are prepared. A recommended negative control for NK cell toxicity assays is the RMA cell line. This cell line is well established MHC-1 deficient target for NK cell killing. Since the primary mechanism of cytotoxicity is mediated by Granzyme B, a recommendation is to determine the baseline expression of Granzyme B. This can be done by harvesting NK cells from the peripheral blood of the mice before activation with poly(I:C) and staining cells for Granzyme B.
In vivo activation of murine NK cells to target cells ex vivo
Once the baseline levels of Granzyme B have been measured, activation of NK cells 24 hours before the killing assay is the next step. poly(I:C) is injected intraperitoneally into each mouse to activate NK cells in vivo. It is essential to start with similar NK cell numbers to normalize the cell percentages when comparing different samples. Once NK cells have been activated, an enrichment step will ensure cell numbers can be standardized and the purity of the sample is similar between groups. Determination of cell viability and concentration is critical for the next step of enriching NK cells. Using fluorescent nuclear dyes like AOPI to stain only the nucleated cells and an automated cell counter to ensure consistent and unbiased analysis of isolated cells will provide the user with consistency, accuracy, and precision in the workflow.

Figure 2: Cellometer K2 fluorescent cell counter provides a simple workflow to determine cell concentration and viability of primary samples within 60 seconds.
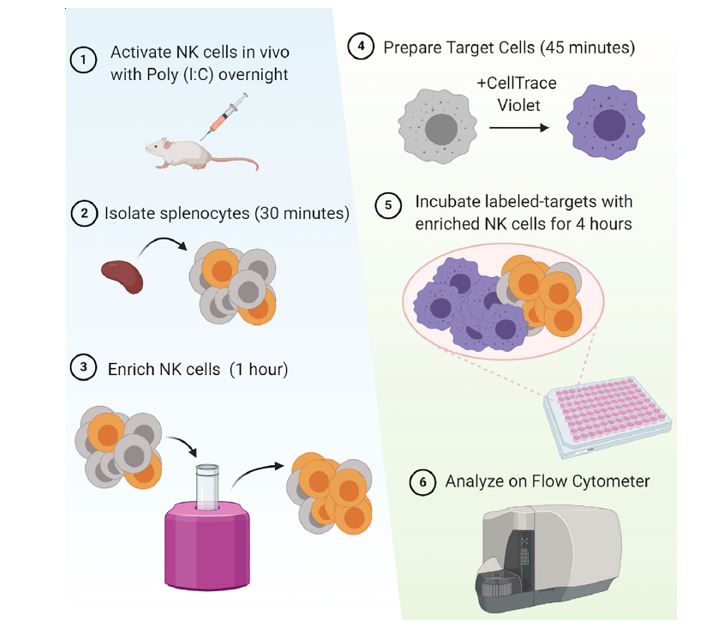
Figure 3: In vivo activation and isolation of murine NK cells. Cells are stained and added to target cells at different E:T ratios to measure killing potency.
Cytotoxicity assay
Cytotoxicity assays can be designed in multiple ways. For instance, various conditions and cell lines can be analyzed. You can use flow cytometry or Image Cytometry to investigate the killing of target cells. Overall, whether using flow-based or image-based cytometry, the common denominator is to determine the percentages of cells dying over time using a viability dye such as 7-AAD, propidium iodide, or DRAQ7, among others. With flow cytometry analysis, the user can use flow cytometry software to gate out debris and only select cells positive for the fluorescent markers that identify their target cells as live and dead. This type of assay can be easily adapted to The Celigo image cytometer, a plate-based high-throughput imaging system that allows the user to monitor the viability of cells multiple times using the same fluorescent nuclear dyes used for flow cytometry. The target cell of choice can be seeded at different effector:target ratios (E:T) in 96 well plates or 384-well plates and loaded into the Celigo image cytometer. A dead nuclear dye can be added to monitor cell death over time, such as propidium iodide, and the experiment is imaged multiple times. The Celigo can scan and analyze a 96-well plate in 5-7 minutes vs. 2-4 hours with flow cytometry. A cell-killing curve is generated to determine the percent specific killing of the NK cells and at what E:T ratio there is most cytotoxicity. One advantage the Celigo provides is the ability to verify the image of the cells while gating for dead cells in real-time, allowing for more accurate identification of dead cells in the sample.
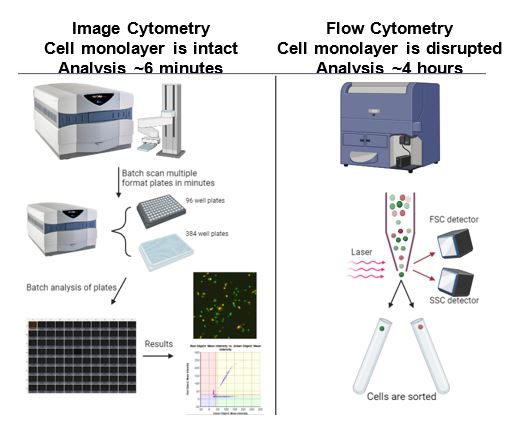
Figure 4: Both techniques have advantages that should be considered as a fit-for-purpose method depending on the information that wants to be extracted. Celigo Image Cytometry allows for high-throughput analysis of cells growing in conditions similar to physiological conditions.
Concluding remarks
Cytotoxicity assays are an essential assay in gene and cell therapy to target cancer cells. Using murine NK cells activated in vivo is another alternative method to perform this type of assay. This study aimed to examine the short-term killing of NK cells when granzyme B mediate cytotoxicity dominates, adding another tool to develop cell therapies against tumors and other malignancies. Learn more about how Celigo applications for Immuno-oncology and other areas can provide ways to streamline and optimize workflows.
References
- Wong, Pamela et al. “Flow cytometry-based ex vivomurine NK cell cytotoxicity assay.” STAR protocols 2,1 100262. 12 Jan. 2021, doi:10.1016/j.xpro.2020.100262
- Albinger, Nawid & Hartmann, Jessica & Ullrich, Evelyn. (2021). Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Therapy. 28. 1-15. 10.1038/s41434-021-00246-w






Leave A Comment