Platelets
Introduction to Platelets
Platelets are often used in the study the clotting factors in blood-based illness. The clotting factors and properties of platelets therefore have a significant implication in cardiovascular and transfusion medicine. Due to their size (~2 microns) they are difficult to visualize and count. By staining them with calcein AM, multiple Cellometer instruments (X2, K2, Vision 10x, Vision and Auto 2000) can identify, image, and count them.
Calcein AM (Calcein acetoxymethyl ester) is a cell permeable, non-fluorescent compound. Upon crossing the cell membrane, calcein AM is rapidly hydrolyzed by cellular esterases inside live cells. The hydrolysis cleaves the AM group, converting the non-fluorescent calcein AM to a strongly green fluorescing calcein. Because the created calcein more hydrophilic it is trapped inside the cells with intact membranes. Cells that do not possess active cytoplasmic esterases are unable to convert calcein AM to calcein, and therefore do not fluoresce green. This allows for a quick and easy detection of metabolically-active cells in a sample.
Calcein AM
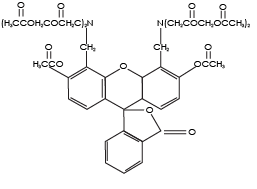
Hydrophobic (readily absorbed by cells)

Endogenous Intracellular Esterases
Calcein
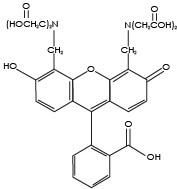
Hydrophilic (remains in the cytosol of cells with intact membranes
Since calcein AM does not require DNA binding, it stains all metabolically-active cells and can be used to measure metabolic activity in non-nucleated cells, such as platelets 1 2. Because calcein AM is photostable, has low cytotoxicity, and does not affect cellular functions, it is a popular stain for the examination of cell vitality and metabolic activity.
Assay Protocol for Platelet Measurement in Whole Blood
Preparation of Calcein AM Reagent
- Pipette 2 µl Calcein AM into 18 µl of dH2O. This is now Calcein AM Solution A. Mix by pipetting up and down at least 15 times.
Staining Procedure for Platelets
- Dilute the platelet sample by adding 5 µl of platelet sample into 85 µl of 1x PBS. Mix well by pipetting up and down.
- Add 5 µl of Calcein AM Solution A to 45 µl of the diluted platelet sample.
- Gently pipette the sample up and down ten times, then incubate for 20 min at 37°C in the dark.
- After the 20 minute incubation, the sample is ready for analysis.
- Gently mix the cell sample by pipetting up and down at least ten times, then load 20 µL into the Cellometer counting chamber and insert into the Cellometer instrument.
- Wait 60 seconds for the cells to settle in the chamber
- Type a name for your sample into the Sample ID text box
- Set the dilution factor to 20.
Detection of Enzymatically Active Platelets Stained with Calcein AM
Bright field image of a diluted whole blood sample
Fluorescent image of Calcein AM stained cells
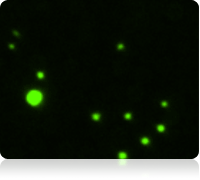
Fluorescent counted image of Calcein AM stained cells
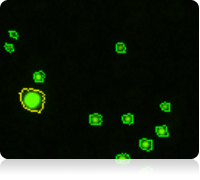
Results
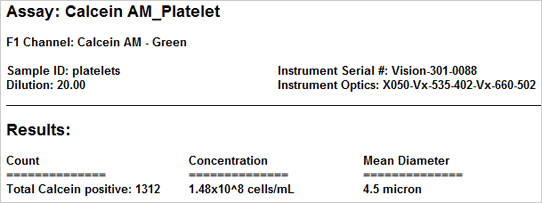
The vitality of the cell sample is measured by staining the cells with calcein AM Once the counting is complete, the Cellometer software automatically reports the total number of counted cells that are calcein AM active, and the concentration of cells. Displayed above are the captured bright-field, captured fluorescent, and the counted fluorescent images from a whole blood sample. Only the white blood cells and platelets are stained with calcein AM Since red blood cells are not stained, they do not interfere with counting. In the counted fluorescent image, counted particles are outlined with a green circle. In this case only the platelets are counted whereas the white blood cells, outlined with a yellow circle, are excluded from counting based on their cell size.
Platelet Bright Field Detection and Concentration Measurement
Laboratories that isolate and work with purified platelet samples can measure the platelet concentration using bright-field imaging. This assay is only recommended for purified platelet samples without red blood cells or other contaminating cellular debris, including platelet-rich plasma (PRP).
Assay Protocol for Purified Platelet Measurement
Sample Preparation
- Dilute the platelet sample by adding 5 µl of platelet sample into 95 µl of 1x PBS. Mix well by pipetting up and down.
- Gently mix the cell sample by pipetting up and down at least ten times, then load 20 µL into the counting chamber.
- Wait 60 seconds for the cells to settle in the chamber.
- Type a name for your sample into the Sample ID text box.
- Set the dilution factor to 20.
Measuring Platelet Concentration Using Bright Field Imaging
Bright field of purified platelets

Counted bright field image
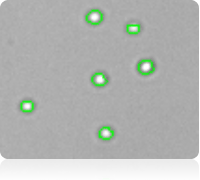
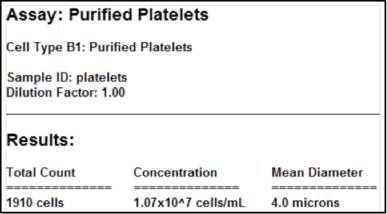
Purified platelets assay results
Conclusions
Platelet counting using Cellometer image cytometry is ideal for platelet containing clinical samples. The ability of the instruments to measure platelet concentration in less than 30 seconds improves counting efficiency and consistency. Platelets can be stained with Calcein AM to determine sample vitality and measure the concentration of enzymatically active platelets in a single assay. To determine which instrument is best for you, contact a Nexcelom specialist today.
Publications Using Cellometer for Platelet Analysis
- Sa Q and Hoover-Plow JL. (2011) EMILIN2 (Elastin microfibril interface located protein), potential modifier of thrombosis. Thrombosis Journal 9(9): 1-8
- Panigrahi S, Ma Y, Hong L, et al. (2012) Engagement of Platelet Toll-Like Receptor 9 by Novel Endogenous Ligands Promotes Platelet Hyperreactivity and Thrombosis. Circulation Research 112(1):103-12
- Zhao Y, Malinin NL, Julia Meller J et al. (2012) Regulation of Cell Adhesion and Migration by Kindlin-3 Cleavage by Calpain. The journal of biochemical chemistry 287(47):40012-20
Publications Utilizing Calcein AM for Platelets Analysis
- Morrell CN, Matsushita K, Chiles K et al. (2005) Regulation of platelet granule exocytosis by S-nitrosylation. PNAS 102(10):3782-7
- Verheul HMW, Jorna AS, Hoekman K, et al. (2000) Vascular endothelial growth factor−stimulated endothelial cells promote adhesion and activation of platelets. Blood 96(13):4216-21
