Determine Cell Viability for Clean and Messy Samples
Viability using Trypan Blue
Ideal for clean samples, the Trypan Blue exclusion assay provides viability by determining dead cells from total cells counted.
Viability using AO/PI
Acridine Orange and Propidium Iodide are nuclear stains able to provide viability measurements in mesey, primary samples.
Comparing Trypan Blue & AO/PI Staining Methods
A head-to-head comparison of viability determined by TB and AO/PI
Induced Necrosis
Measure induced necrosis by staining your cell sample with different membrane exclusion dyes
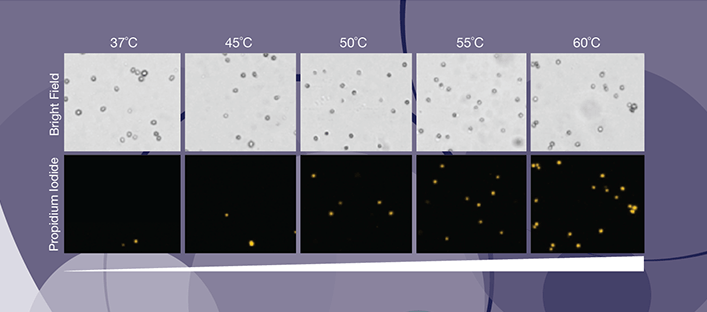
Viability Measurements using PI
Directly measure the number of dead cells and percent viability by staining your samples with propidium iodide.
Alternate Membrane Exclusion Dyes
Using 7AAD, SYTOX Green, Ethidium Bromide, SYTOX Red, DRAQ7 and DAPI for viability measurements.