Quantify Invasion into Matrigel
Measure the Cell Invasion from a Spheroid into Matrigel
- Directly image tumor spheroids in various microwell formats
- Non-invasive bright field imaging allows the user to image the same plate over multiple days
- Perform a two-color fluorescent viability assay
Introduction
The Celigo imaging cytometer has been developed to fully automate live cell analysis of tumorspheres. This automated morphometric analysis tool significantly reduces the time and effort needed to quantify key aspects of 3D spheres including size, growth, growth tracking over time and response to chemotherapeutics.
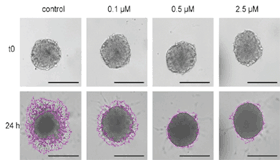
Reduced Cell Invasion from a U-87 MG 3D Spheroid into Matrigel in the Presence of 17-AAG
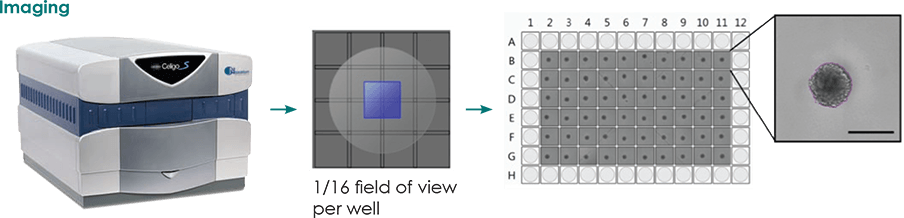
Experimental Setup
- Day 0-4: form spheroids
- Day 4: add Matrigel to provide a semi-solid gel-like matrix
- Day 4-7: use Celigo imaging cytometer to determine the area occupied by individual cells or cell clusters
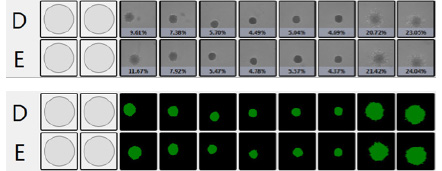
Bright Field Spheroid Images
U-87 MG + 17-AAG
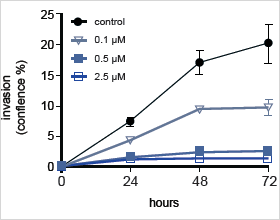
Measure Cell Invasion from 3D Spheroid into Matrigel
Pre-Matrigel
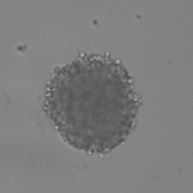
Bright Field Spheroid
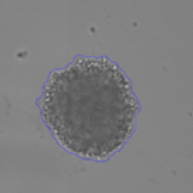
Bright Field Identified Spheroid
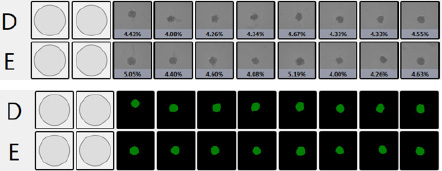
Percent confluence and represented fill view as seen in the Celigo software
Measure Cell Invasion from 3D Spheroid into Matrigel
48 hr after the addition of Matrigel
Bright Field Spheroid
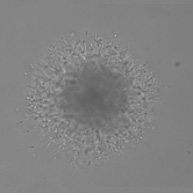
Bright Field Identified Spheroid
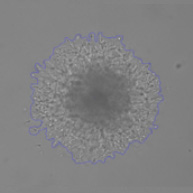
Percent confluence and represented fill view as seen in the Celigo software