Cell Analysis for Breweries and Biofuels
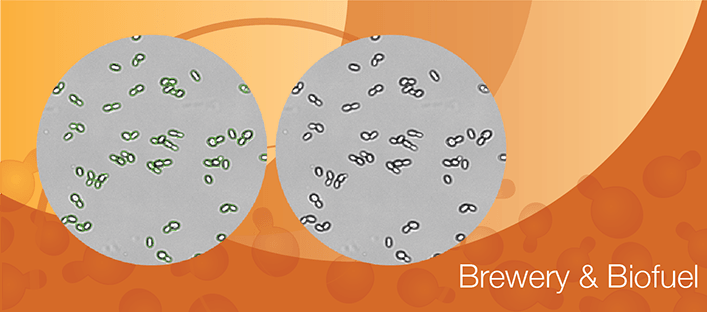
Yeast in Brewing
How brewing yeasts are counted and analyzed for viability in bright field and fluorescent imaging using PI, Oxonol and AO/PI viability staining.
Ale and Lager Yeast
Examining Saccharomyces cerevisiae for viability and vitality using the Cellometer.
Yeast in Biofuel
How complex mediums such as corn mash or sugar cane can be analyzed for concentration and viability.
Advanced Yeast Analyses
Examining how the Cellometer systems can determine vitality, glycogen content, neutral lipid content, trehalose content and perform GFP transfection.