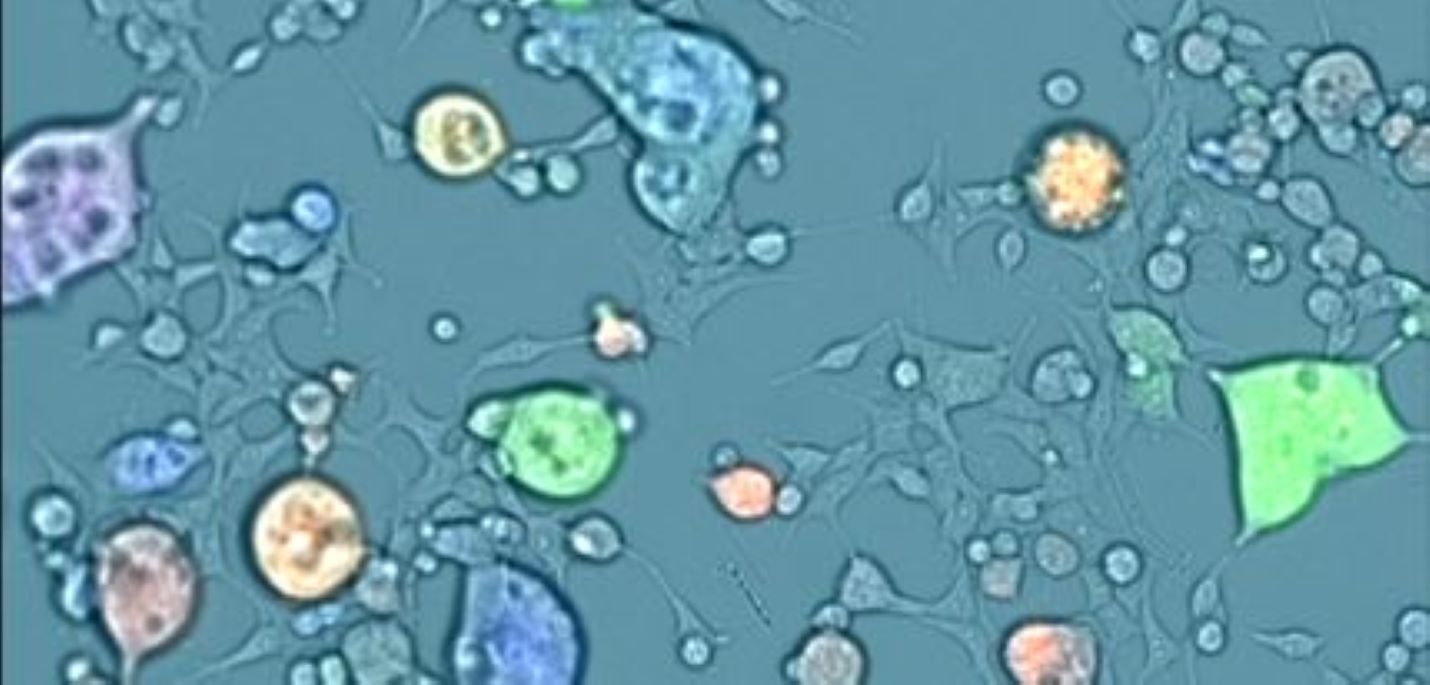
What is image cytometry?
Introduction
Cell-based technologies have advanced rapidly in the decades since the invention of the first microscopy instrumentation in the 17th century.1 Advancements in cell-based detection technologies, cell-modeling software, and novel cell interrogation reagents and assays have significantly increased our understanding of the inner workings of cells. These technological innovations have propelled rapid and novel therapeutic developments due to improved speed, quality, and throughput.
What is cytometry?
Cytometry is the measurement of the properties of cells such as concentration, size, morphology, surface proteins, and nucleic acid content amongst others. It can be as simple as counting the number of viable and nonviable cells with a standard light microscope or as complex as measuring protein expression using flow cytometry with ten lasers and twenty biomarkers.2 One of the most performed cell-based assessments is the manual counting of cells using a hemacytometer under a light microscope. This laborious method can be extremely time-consuming, has low-throughput and high operator-dependent variation, and can result in unreliable data. To address this, there are currently innovative methodologies that can reduce assay time and improve cell counting precision to generate high-quality results.
What is image cytometry?
As the name suggests, image cytometry is a method to assess cell characteristics via imaging techniques. With the advancements in digital cameras, light-emitting diodes (LEDs), microfluidics, image-based fluorescent reagents, and image analysis algorithms, the popularity of image cytometry has increased over the last decade. Image cytometry combines the use of microfluidics to hold cell samples and advanced imaging technology to quantify cell concentration, morphology, and brightfield or fluorescent-based biomarker expression.
What is flow cytometry?
Flow cytometry is a method in which the cells flow in a high-velocity controlled single-stream fluidic system and are interrogated with electrical or optical stimulation. Flow cytometry can quantify both morphological and biological attributes of distinct types of cells. By exciting each cell in the fluidic system with different laser sources to detect reflected light or emitted fluorescence. These fluorescently labeled biomarkers and the physical properties of the target cell sample are evaluated to determine specific populations of interest. Flow cytometers can be extraordinarily complex and may require trained specialists to perform the various functions necessary to generate the best results.
Flow cytometric applications such as heterogeneous population analysis, DNA sequencing, immunophenotyping, and other functionalities for a myriad of cell-based assays are performed daily from general research to high-dimensional parametric analysis. Typically, the multi-color system requires single stain controls for setting up compensation to eliminate fluorescent crosstalk into different channels. Although flow cytometry is sensitive and can be fast for large samples, it has inherent issues. For example, the system typically requires a long start-up and shut-down process, the fluidics can clog with cell samples, and it requires washing steps between each sample.
Advantages of image cytometry
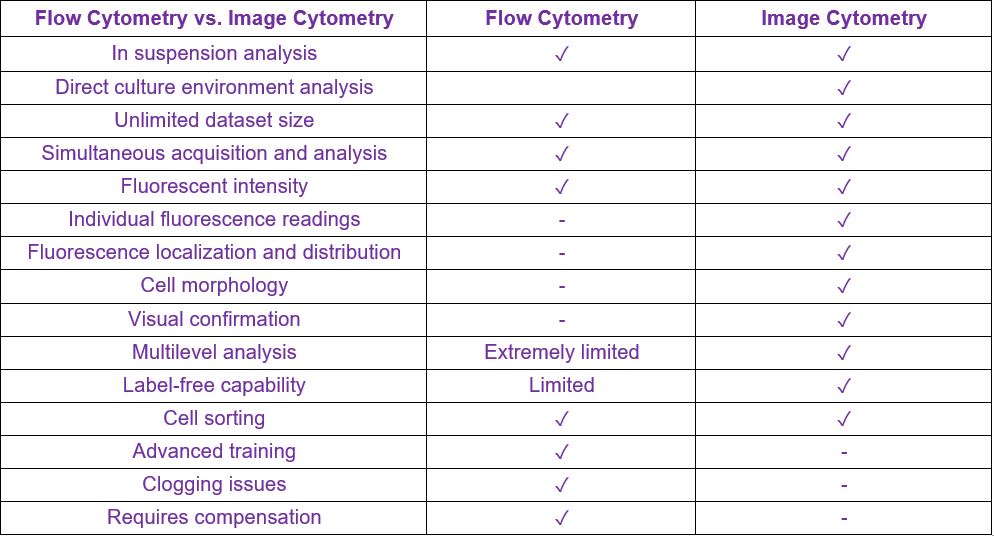
Image cytometry can rapidly acquire brightfield and fluorescent images in a high-throughput manner without the need to perform time-consuming start-up and shut-down procedures. The fluorescent filters can be specifically designed to eliminate the need for fluorescence compensation. Like flow cytometers, image cytometers can capture fluorescence intensities from the target cells and generate fluorescence histograms and scatter plots to determine specific cell populations such as viable, nonviable, or various biomarkers. Unlike flow cytometry, image cytometry can acquire both brightfield and fluorescent cell sample images for cytometric analysis, and a highly sophisticated image analysis algorithm is used to automatically segment each cell and directly measure fluorescent intensities from each dye. Overall, the acquired images provide both visual confirmation and correlation between cells, and recording the individual fluorescence minimizes data uncertainties. In addition, image cytometry systems do not contain any fluidics, eliminating clogging issues and making the methodology more robust.
Image cytometry applications
Using the appropriate assays and image analysis software, image cytometers can measure specific cell characteristics for different biological applications. Image cytometry can be employed to perform simple cell count and viability measurement for peripheral blood mononuclear cells (PBMCs), five to six fluorescent biomarkers for immunophenotyping, protein expression, cell health analysis such as apoptosis, mitochondrial potential, reactive oxygen species (ROS), and cell cycle, as well as more complex assays such as cell painting for high content image cytometric analysis. Image cytometry can perform these routine applications described above and alleviate the burden on flow cytometry.
What can one see with image cytometry?
- Total and individual cell counts (Cell concentration)
- Dying and damaged cells (Apoptosis)
- Live, viable cells (Viability)
- Adherent cells
- Cells that exhibit specific surface markers (Immunophenotyping)
- Morphological and structural information
- Multilevel identification and analysis
- Cell size
Automated image cytometry brings cell analysis back to the bench for readily available and reliably accurate cell data and tracking to streamline cell-based workflows. Image cytometry instruments are easy to use and do not require advanced training or a core facility to receive the same format of data as flow cytometry. This advanced technology saves time and energy, achieving faster cell characteristic information and viability data while maintaining precise cell information.
References
- Mandal, Ananya. “Flow Cytometry History”. News-Medical. https://www.news-medical.net/life-sciences/Flow-Cytometry-History.aspx. (Accessed June 21, 2023).
- Nolan, JP. The evolution of spectral flow cytometry. Cytometry. 2022; 101(10) 812– 817. https://doi.org/10.1002/cyto.a.24566





Leave A Comment