Colorectal cancer (CRC) is the second leading cause of cancer mortality and accounts for nearly 8% of all new cancer cases in the United States [1]. Many common chemotherapeutic treatments combat disease progression by inducing replication stress (RS) to impair replication. The ATR-CHK1 pathway plays a critical role as a replication checkpoint and is a promising target for emerging therapies [2]. Microsatellite instability tumors (MSI+) in patients with CRC present with a frameshift mutation in the ATR gene in ~40% of cases yielding an altered RS response. Recently, Egger et al. investigated the effect of this mutation in a novel isogenic cell model of HCT116 cells (CRC, MSI+ cell line). The investigators tested a panel of drugs using sulforhodamine survival assays (SRB) to identify candidate molecules exhibiting increased sensitization on the ATR mutant cell line
Cytotoxicity Measurements
The Celigo Imaging Cytometer was used to differentiate between cytotoxic and cytostatic effects of SN-38 and VE-822 in living cells over five days when exposed to the respective IC50 doses for each molecule. Two molecules identified during the drug screening process, SN-38 (topoisomerase I inhibitor) and VE-822 (ATR inhibitor), demonstrated the most pronounced effects with a twofold decrease in the IC50 in the mutant HCT116 cells compared to the wildtype controls.
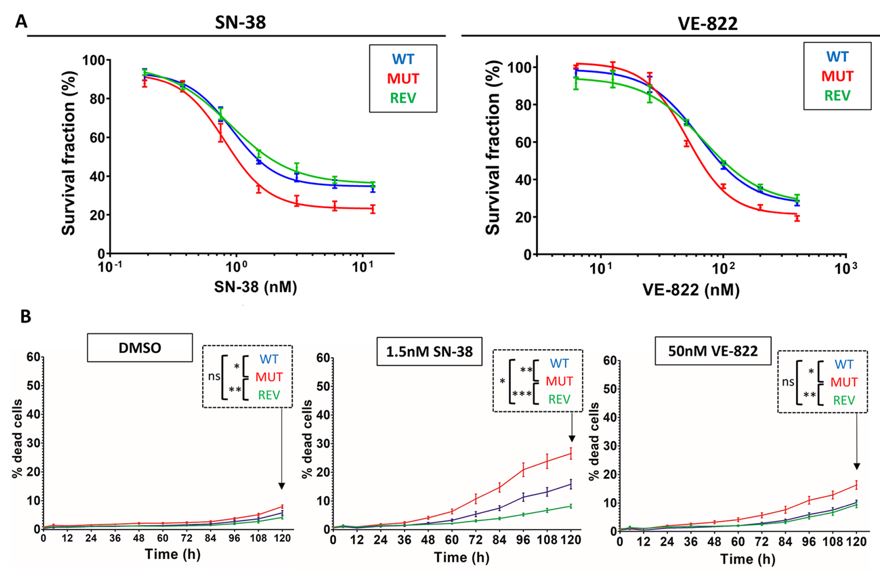
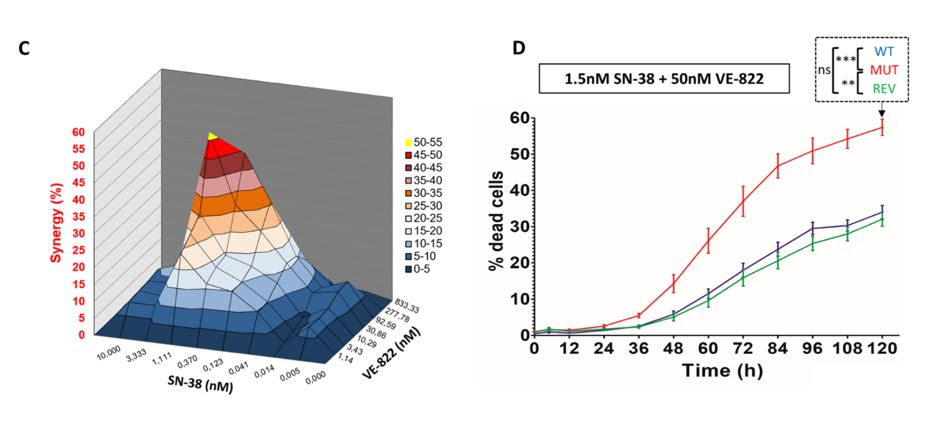
Both molecules were also combined for a 72-hour survival test to explore any synergistic effects. The approximate peak synergistic dose was the IC50 dose for each drug independently. The combination protocol yielded significantly higher cytotoxic effects on the mutant cell lines, revertant clones, and wild-type cell lines. This finding underscores the sensitizing effects of each molecule on the mutant ATR cell line and the overall impact of targeting replication checkpoint pathways as a treatment strategy.
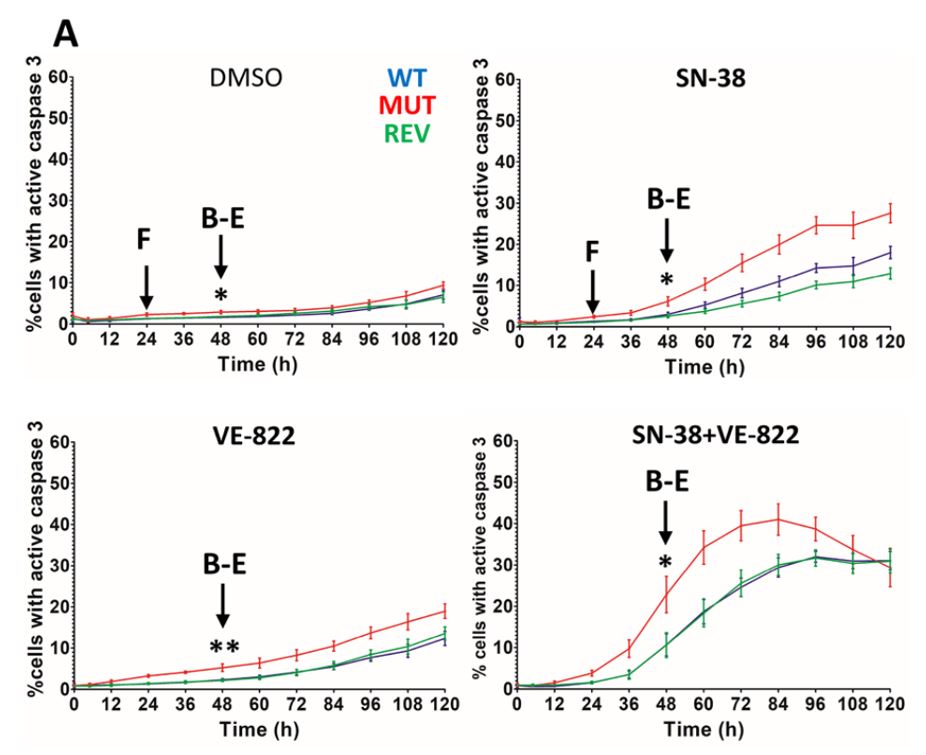
Apoptosis Induction Measurements with Caspase 3/7
The cytotoxic screening assays provided a reliable method of investigating the percentage of viable cells within the population following a specified treatment protocol. As an indication of apoptotic induction, Caspase 3 activation was measured on the Celigo system to provide more insight into the cytotoxic effects of SN-38 and VE-822 using the Caspase 3/7 kit from Nexcelom Bioscience. Mutations to the ATR gene yielded a significant increase in apoptosis induction, with the combined treatment applied to the mutant cell line producing the most pronounced effect.
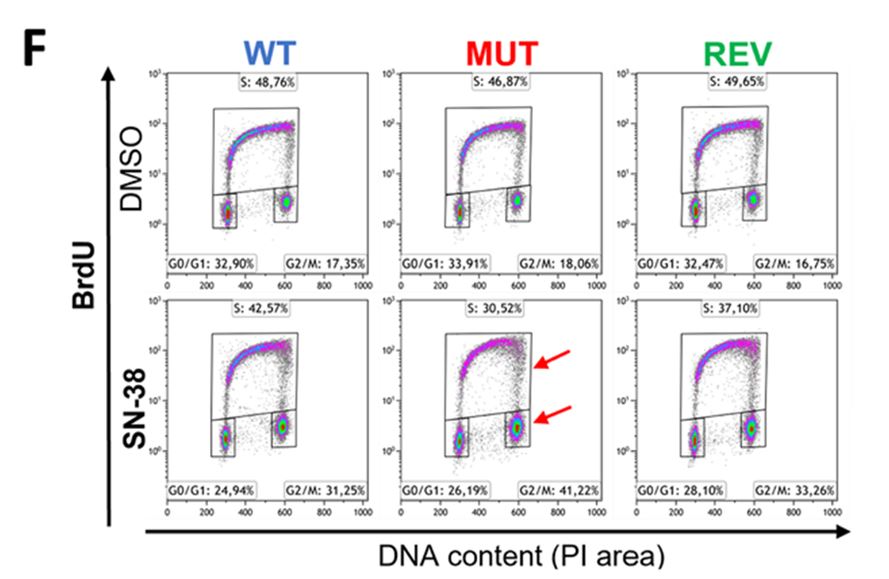
DNA damage was measured using a combination of western blots, immunofluorescence microscopy, and flow cytometry. Collectively, these data suggest that the ATR mutant cell line was not capable of fully activating the ATR-CHK1 pathway. The flow cytometry data in the SN-38 group demonstrated a reduction in the percentage of replicating cells and a pronounced accumulation of cells in the G2/M phase. The Celigo system rapidly performs cell cycle analysis on adherent cells with a variety of pre-defined protocols (Benefits of using Celigo for Cell Cycle Analysis). The flexible gating interface of the Celigo software is used to identify and quantify populations of cells in each phase of the cell cycle. This further investigation supported a causal link between the failure of the replication checkpoint and the measurable increase in apoptosis measured on the Celigo.
Replication checkpoints in cancer cells are an appealing target for emerging therapies due to their key role in driving growth. Mutations, such as the ATR mutation in CRC, offer insight into the complex pathways governing the proliferation of cancer cells. It also offers an opportunity to explore novel treatment protocols to circumvent innate protective mechanisms that reduce the efficacy of traditional chemotherapies.
The Celigo Image Cytometer offers a wide variety of applications for automated cell counting and cell-based assays with intuitive software analysis tools and automation options to accommodate high-throughput needs. Learn more about cellular models and the direct cell counting assays on our Celigo Image Cytometer Applications page or contact us directly to book a seminar or live demonstration today.
References
- Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics. CA Cancer J Clin 70, 145–164. https://doi.org/10.3322/caac.21601 (2020)
- Rundle, S., Bradbury, A., Drew, Y. & Curtin, N. J. Targeting the ATR-CHK1 axis in cancer therapy. Cancers (Basel) https://doi.org/10.3390/cancers9050041 (2017).





Leave A Comment