Therapeutic Potential of HIV Protease-Activable CASP3
Authors: Kosuke Miyauchi, Emiko Urano, Mari Takizawa, Reiko Ichikawa, Jun Komano
Scientific Reports 2012; 2: 359; doi:10.1038/srep00359.
Background: As the primary executioner caspase involved in apoptosis, Caspase-3 is a key therapeutic target in cancer cells exhibiting uncontrolled cell proliferation. Because Caspase-3 activation is highly regulated, development of a viable treatment method has been difficult. Recent gene therapy procedures have been effective against HIV-1 infection, but development of treatment resistance due to mutation is a problem. Negative effects on healthy cells is also a problem.
Purpose: To inhibit cancer cell growth and propagation of the human immunodeficiency virus-1 (HIV-1) using a genetically-engineered mutant of caspase-3, CASP3*, to initiate apoptosis utilizing a lentivirus-like nanoparticle (LENA) for delivery to target cells.
Methods: The mutant CASP3* was designed with a cleavage site for the HIV-1 protease. CASP3*-gag-pol and VSV-G (Vesicular stomatitis virus G) expression vectors were co-transfected into 293T cells. VSV-G-encapsilated LENA containing activated CASP3* protein was expressed. Mammalian cells were incubated with CASP3*-LENA-containing medium. The VSV-G facilitated binding of CASP3*-LENA to cells and entry via endocytosis. LENA content (active CASP3*) was released into the cell cytoplasm.
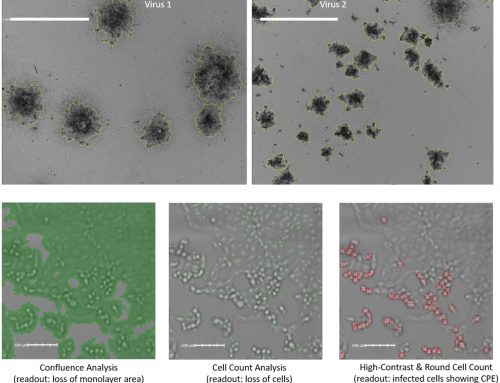
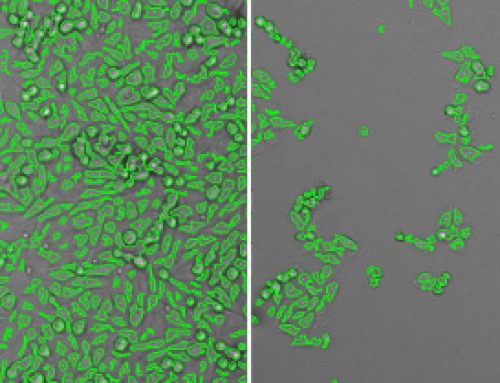
Results: The acute T cell lymphoblastic leukemia cell line MOLT-4 lost 93% of cell viability one day following CASP3*-LENA exposure. Incubation with a VSV-G inhibitor or cell-permeable CASP3 inhibitor protected cells from apoptosis, showing dependence on both VSV-G function and the proteolytic function of CASP3* for cell death. CASP3*-LENA also induced apoptosis in four other cells lines, including leukemia and neuroblastoma cells.
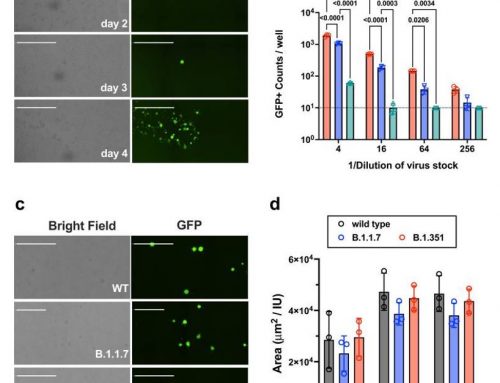
Testing of SUP-T1/CASP3* cells exposed to HIV-1 for Annexin V binding showed that CASP3* induced apoptosis upon HIV-1 infection. Activation of CASP3* by viron-associated HIV-1 protease led to depletion of virus-infected cells from the culture by apoptosis and a reduction in HIV-1 replication.
Findings of Note: Many cancer cell lines, such as MT-4 cells, express intracellular caspase inhibitors, making them highly resistant to apoptosis. It may be possible to design a CASP3* mutant that does not interact with a cellular inhibitor of apoptosis protein (CIAP), making the treatment more effective against apoptosis-resistant cancer cells.
Cell killing activity of CASP3*-LENA was not observed in peripheral blood mononuclear cells, indicating that cancer cells may be more susceptible to CASP3*-LENA delivery and induction of apoptosis.
Author’s Conclusion: Delivery of a CASP3* mutant activated by HIV-1-encoded aspartate protease using the lentivirus-like nanoparticle (LENA), is a viable platform for the development of a CASP3*-based therapy against lymphoid malignancies and HIV-1 infection. As an HIV-1 therapy, this method would offer reduced “off-target” effects and would not yield latently-infected cells, overcoming some of the shortcomings of current therapies.
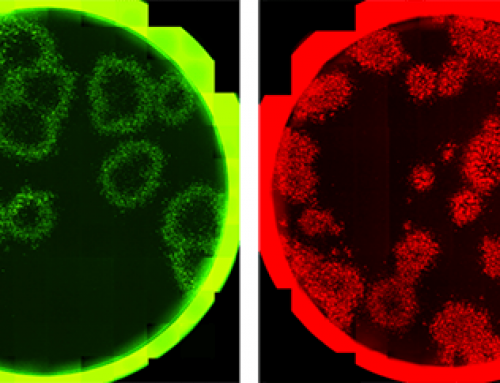
Reviewer’s Comments: This study highlights the importance of apoptosis and the caspases in therapeutic research for both oncology and HIV-1 infection and the importance of cell-based assays in clinical research. Though these researchers utilized other methods, analysis of cell viability, Annexin-V binding and Caspase-3 activity can be easily performed with the Cellometer Vision CBA Image Cytometry System.

Leave A Comment