High-throughput screening of patient sera for pre-existing AAV vector antibodies
Pre-existing neutralizing antibodies (nAbs) pose a serious barrier to safe and effective gene therapy use. It has been reported that approximately 70% of adults have sera antibodies for AAVs (adeno-associated viruses) and memory T cells [27]. A recent study investigated the prevalence of nAbs against AAV-1, -2, -5, and -8 in blood samples from healthy donors. The results were troubling as they found that 47-74% of samples had AAV-2 nAbs even though low titers can block in vivo transduction [28].
Though viral genomic material has been removed, the capsid carrying the therapeutic payload can trigger an immune response. Prior viral exposure can lead to undesired humoral and/or T cell-mediated immunity that will be reactivated upon vector delivery [29-32].
- Neutralizing Antibody Screening
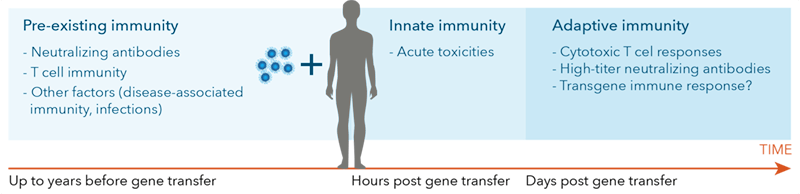
Figure 1. Wild-type AAV exposure and patient characteristics determine an individual’s response at vector delivery and overtime.
It is critical to assess immune status in patients to monitor gene therapy efficacy [33]. Subjects previously treated with gene therapy may require a second dose as an initial treatment may have activated a patient’s adaptive immune system to eliminate the vector upon reintroduction. This proves that assessing a patient’s immune status is critical to monitoring gene therapy efficacy [33].
To improve the effectiveness of vector delivery, image cytometers can be employed for:
- Rapid screening for nAbs prior to treatment
- Cell-based in vitro transduction inhibition assays
- Long-term patient monitoring
- Humoral immune responses for antibodies against the vector and gene therapy protein product
- T-cell immune responses
Use plate-based imaging to screen patient sera for neutralizing antibodies and monitoring immune responses over time
The Celigo Image Cytometer is a plate-based high-throughput system that simultaneously images and analyzes cells in standard multi-well plates using brightfield and fluorescence technology. The Celigo rapidly screens for sera nAbs that can neutralize AAV vectors for potential pre-existing immunities.
- High-throughput automated imaging and direct analysis in 96-well plates
- No trypsinization necessary to count cells
- Measure antibody neutralization effects in less than ten minutes
This section provides two technical examples of how the Celigo Image Cytometer can be used to develop a robust screening assay for potential pre-existing immunity to AAV vectors.