Whole-well imaging analysis for quality control of cell-based assays in AAV vector design
Extensive cell characterization is critical for gene therapy research and development. Accurate and precise cell characterization is necessary for optimizing experimental conditions, manufacturing regulation, and eventual product release. To ensure precise results are produced, cell-based assay conditions must have quality control.
The AAV vector development process is complex and tedious (Figure 1). To accurately perform high-quality cell-based assays many factors must be optimized to avoid end-product variations including host-cell quality, quantity, viability, passage number, and cell preparation procedures.
What parameters should be monitored for optimizing the process to produce AAV2-transgene vectors?
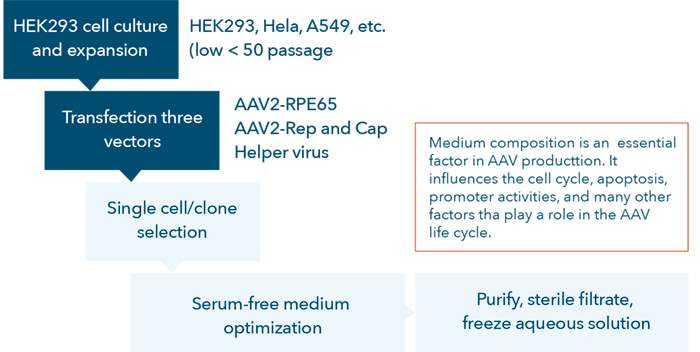
Figure 1. AAV vector development process flow chart.
During AAV vector development, researchers commonly utilize luciferase and GFP reporters in place of the transgene to investigate the expression level after transduction. After performing appropriate characterization experiments, the reporters are replaced with the transgene to produce the actual AAV vector products [12, 23-25]
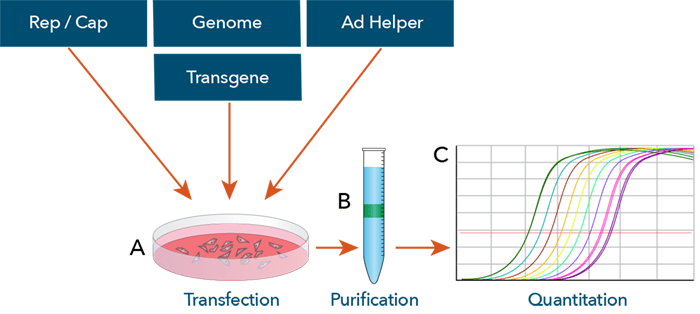
The adeno-associated virus (AAV) vector production process. (A) The AAV vector production starts with the transfection of mammalian cells with 3 plasmids (Cap proteins, transgene genome, and adenovirus genes for replication) typically with HEK293 cells. (B) The transfected cells are then collected and lysed after 48 – 72 h, where the vectors are then purified. (C) Finally, the vectors are quantified by RT-PCR. doi:10.1128/microbiolspec.MDNA3-0052-2014.f3
Currently, researchers culture targeted cells (e.g., HEK293, HeLa, A549, etc.) at a low number of passages (<50), seed them in standard multi-well microplates and transfect the target cells with the AAV vectors for testing. During the first two steps indicated in the flow chart (Figure 1), quality control for the development of AAV vectors, it is important for researchers to:
- Ensure cell seeding uniformity within plates and wells
- Generate accurate confluence or cell count measurements in standard multi-well plates after cell seeding
- Confirm that passaging target cells do not impede cell proliferation for downstream cell-based assays
- Ensure cell-based assays are robust and cell washing procedures can retain the number and health of the target cells in the well to support vector transduction
- Fully lyse cells to generate the proper detection signals
Image cytometry enables rapid, label-free cell analyzation to minimize cell-based assay variability
The Celigo Image Cytometer is a high-throughput, plate-based system that rapidly images and analyzes cells in standard multi-well plates using both brightfield and multichannel fluorescence. The Celigo provides quality control of cell-based assays for AAV vector research and development.
- Image and analyze 96- and 384-well plates in brightfield in less than five minutes per plate
- Rapid whole-well imaging to monitor cell quality
Three technical examples of how the Celigo Image Cytometer supports optimization of cell-based assays for AAV vector design.