Modern Cell-Based Assay Principles for Gene Therapy Development
An overview of how Nexcelom solutions and associated technologies remove barriers in the application, optimization, and refinement of gene therapies.
The overarching goal of gene therapy is to modify gene expression to treat disease [1] [https://www.asgct.org/education/more-resources/gene-and-cell-therapy-faqs]. Researchers first began to think about how modification could be achieved as the first molecular biology techniques were developed in the 1970s [2-4]. These cutting-edge therapies deliver functional genetic payloads, which is very different than administering a known quantity of a drug. Providing a precise gene dose to the affected cells in the patient’s body is paramount to safety and efficacy, and requires accurate quantification and thorough preclinical testing. Furthermore, moving from the bench to the clinic requires extensive immunogenicity testing, as every component of a gene therapy product from the vector to cargo to therapeutic protein can elicit an immune response. Gene therapy is regulated by the U.S. Food and Drug Administrations’ Center for Biologic Evaluation and Research. More information on FDA guidance is available here [5-11].
There are three main approaches to gene therapy:
- Replacing the mutated gene with a normal copy
- Inactivating the mutated gene to prevent it from causing more harm
- Introducing a new gene to treat a disease
Gene therapy is accomplished through in vivo or ex vivo therapy
Broadly speaking, gene therapy is ex vivo or in vivo [12, 13]. Ex vivo gene therapy involves harvesting cells from the patient, modifying the DNA, expanding the modified cells, and infusing them back into the patient. In vivo gene therapy is when the targeted cells are altered inside the body and the gene therapy is administered directly to the patient. This approach is more technically challenging because it requires targeted delivery of the payload to the affected cells and or tissues, where the gene must be effectively expressed. Gene therapy utilizes four broad categories to combat these issues: genetically modified cell-based immunotherapies, viral vectors, non-viral vectors, and gene editing [17-20].
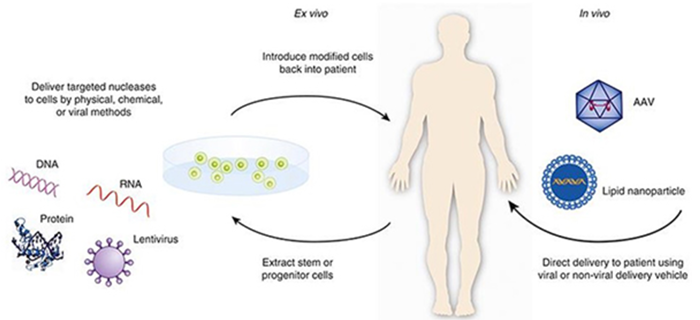
There two major types of gene therapies: Ex Vivo and In Vivo. Image credit https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
Of the four main types of gene therapies, viral vectors are incredibly efficient at transfecting or transducing host cells. For this reason, up until 2017, ~70% of the 3000+ clinical trials conducted employed viral vectors [21].
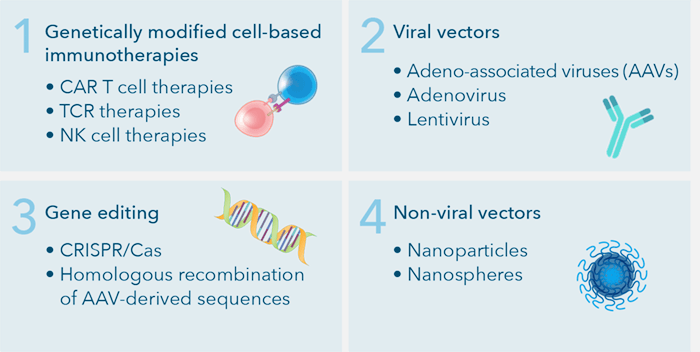
Four main types of gene therapy: Genetically modified cell-based immunotherapies, Viral vectors, Gene editing, and Non-viral vectors
In general, viral vector-based gene therapy development follows several relatively complex workflows [22]. First, gene therapy products are unique in that they are produced in mammalian cells, so it is critical to select and isolate suitable target cells during preclinical research and development. Next, the appropriate vectors are selected based on the following considerations:
- DNA load capacity
- Transduction efficiency
- Cell tropism
- Target cell mitotic status
- Geno-/cytotoxicity
- Limited to no immune response in humans
Subsequently, assay development is required to determine the transduction efficiency to ensure the proper gene is transduced into the selected cell types. Finally, due to safety concerns, researchers are required to screen vector antibodies in patient sera to test for transduction inhibition.
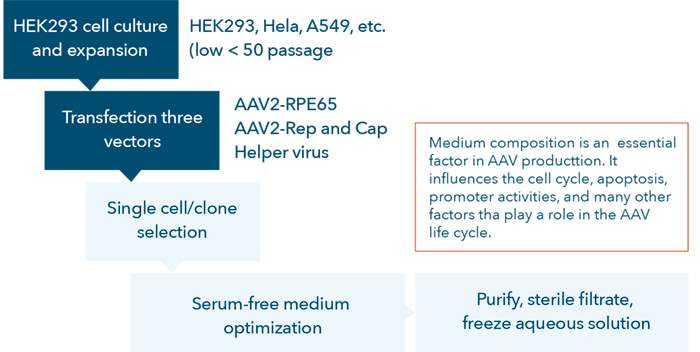
AAV vector development process flow chart.
Current technologies for gene therapy research and development
Many technologies and methodologies are employed for gene therapy research and development such as PCR, cell-based assays, LC-MS, Flow Cytometry, ELISpot, etc. Cell-based assays are one of the most commonly used methodologies for gene therapy development in academics and industry.
These existing technologies also face various challenges:
- Sensitivity
- Robustness
- Sample availability for method development and validation
- Selectively
- Sample availability for all analyses required
- Sample matrix type
- Sample stability
Viral vector-based gene therapy research can encounter challenges when developing and optimizing cell-based assays:
- Designing effective vectors
- Increasing transduction efficiency
- Optimizing culture conditions
- Banking cells
- Measuring gene expression
- Scaling up production
- Effective delivery to cells in vivo (due to pre-existing neutralizing antibodies)
Image cytometry assays improve speed and efficiency for cell counting and cell-based assays
Nexcelom’s image cytometry and automated cell counting systems support three important aspects of viral vector-based gene therapy research and development. First, we provide rapid, user-friendly solutions for cell counting, measuring transduction efficiency, proliferation, viability, cytotoxicity, apoptosis as well as other biological phenomena to support researchers developing new gene therapies. Additionally, image cytometry methods can be used to test patient samples for pre-existing antibodies that may prevent the therapy from being effective and to help monitor immune responses during treatment.
- Quality control to develop high-quality cell cultures
- Rapid cell counting
- Accurate assessment of proliferation and viability
- Cell culture condition optimization
- Cloning selection and verification
- Selecting and fine-tuning an appropriate AAV vector
- Simultaneously assess transduction efficiencies and expression level
- Developing screening assays to identify pre-existing antibodies in patients
- Screen patients for vector-neutralizing antibodies
- Cell-based in vitro neutralization assays
Overall benefits of employing image cytometry for cell counting and cell-based assays
- Perform label-free cell proliferation assays in 96- and 384-well plates at 5 min per plate
- Direct cell counting to determine transduction efficiencies and expression level from 6- to 1536-well plates
- High speed, high-throughput, whole-well imaging for quality control of cell-based assays
- Cell-based antibody binding assays in 96-well plates in 15 minutes with four fluorescent colors
- High-throughput cell counter provides precise cell count and viability for 24 samples in less than three minutes
Gene therapy research and development can utilize Nexcelom image cytometry and automated cell counting systems and to focus on key improvement areas for cell-based assays and cell counting.
Improve Research, Efficiency, and Quality of Product Development
Cell-based assays are highly important for gene therapy research and development. Image cytometry and automated cell counting systems are utilized to optimize cell-based assays.
- Cell quality
- Cell count, viability, and apoptosis by caspase 3/7 or Annexin V can confirm the quality of the cells before experimentation (measured by Cellometer Spectrum, Auto 2000, or K2)
- A large number of samples can be quickly assessed (24 samples in <3 min measured by Cellaca MX)
- Advanced cell-based assays such as cell proliferation, cell cycle, apoptosis, and senescence can be performed in 96- or 384-well plates (measured by Celigo)
- Cell culture condition optimization
- Media and reagent optimization can be measured by assessing cell proliferation within wells in 6- to 96-well plates (measured by Celigo)
- High-throughput cell counting is needed (measured by Cellaca MX) before seeding cells to assessing multiple conditions
- Transduction and expression efficiency
- Transduction efficiencies can be measured by direct cell counting of total and fluorescent-positive cells (measured by Cellometer Spectrum and Celigo)
- Expression level can be determined by measuring fluorescent intensities in genetically modified cells (measured by Cellometer Spectrum and Celigo)
- Single-cell clone verification
- Whole-well imaging is required to ensure single-cell monoclonality and can be quickly determined through brightfield colony outgrowth or directly using fluorescence (measured by Celigo)
Screen pre-existing antibody to AAV in sera
- An antibody or serum-based neutralization assay
- Pre-existing immunity to viral vectors can lead to neutralization and requires a cell-based in vitro transduction inhibition assay for screening (measured by Celigo)
Monitor immune responses
- Humoral immune response by ELISAs of serum levels for vector-
- Requires accurate PBMC enumeration for ELISA analysis (measured by Cellaca MX)
- T cell immune responses by ELISpot IFN-γ test
- Requires accurate PBMC enumeration for ELISpot analysis (measured by Cellaca MX)
Learn more: