Automation Method to Increase Efficiency in Cell Line Development
Sarah Kessel, Leo L. Chan, and Olivier Dery
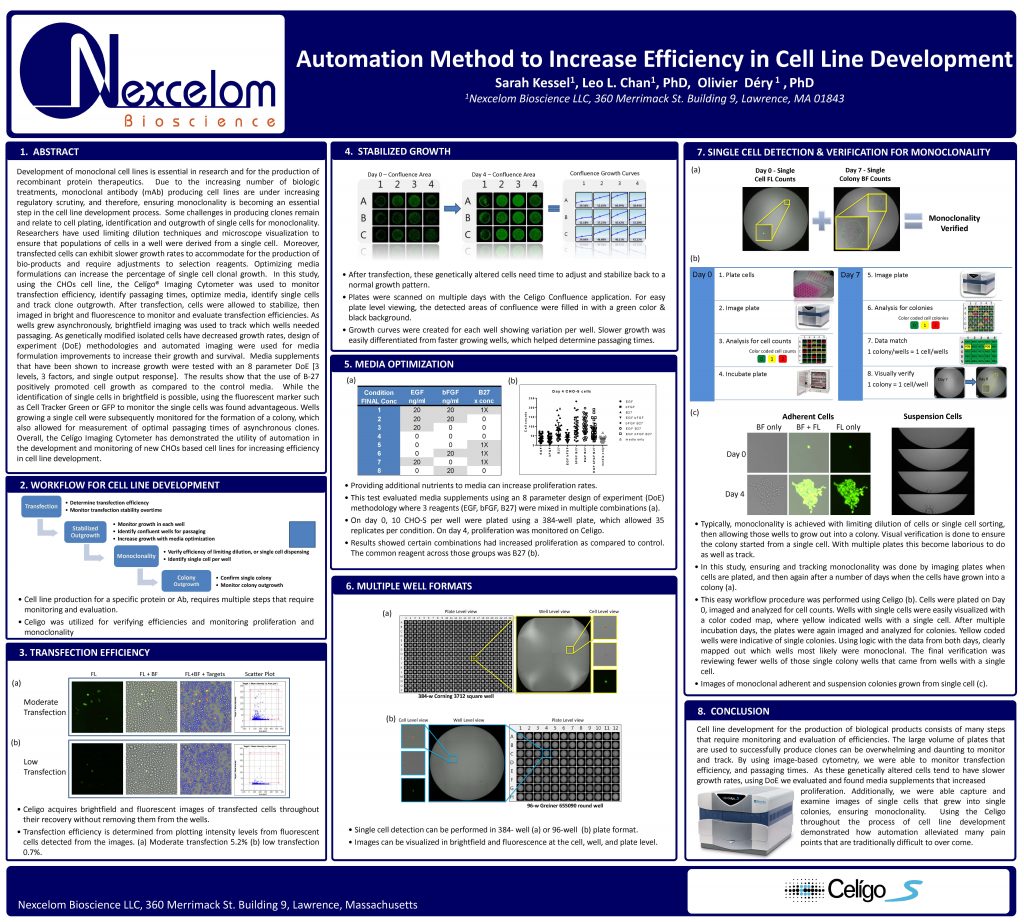
Development of monoclonal cell lines is essential in research and for the production of recombinant protein therapeutics. Due to the increasing number of biologic treatments, monoclonal antibody (mAb) producing cell lines are under increasing regulatory scrutiny, and therefore, ensuring monoclonality is becoming an essential step in the cell line development process. Some challenges in producing clones remain and relate to cell plating, identification and outgrowth of single cells for monoclonality.
Researchers have used limiting dilution techniques and microscope visualization to ensure that populations of cells in a well were derived from a single cell. Moreover, transfected cells can exhibit slower growth rates to accommodate for the production of bio-products and require adjustments to selection reagents. Optimizing media formulations can increase the percentage of single cell clonal growth. In this study, using the CHOs cell line, the Celígo® Imaging Cytometer was used to monitor transfection efficiency, identify passaging times, optimize media, identify single cells and track clone outgrowth. After transfection, cells were allowed to stabilize, then
imaged in bright and fluorescence to monitor and evaluate transfection efficiencies. As wells grew asynchronously, brightfield imaging was used to track which wells needed passaging. As genetically modified isolated cells have decreased growth rates, design of experiment (DoE) methodologies and automated imaging were used for media formulation improvements to increase their growth and survival. Media supplements that have been shown to increase growth were tested with an 8 parameter DoE [3
levels, 3 factors, and single output response]. The results show that the use of B-27 positively promoted cell growth as compared to the control media. While the identification of single cells in brightfield is possible, using the fluorescent marker such as Cell Tracker Green or GFP to monitor the single cells was found advantageous. Wells growing a single cell were subsequently monitored for the formation of a colony, which
also allowed for measurement of optimal passaging times of asynchronous clones. Overall, the Celígo Imaging Cytometer has demonstrated the utility of automation in the development and monitoring of new CHOs based cell lines for increasing efficiency in cell line development.