Cellometer K2 Fluorescent Cell Counter
Advanced Cell Counter with Option for 21 CFR Part 11 Module

“This instrument [Cellometer K2] is definitely worth the investment.”
“This machine [Cellometer K2] has transformed the way I culture.”
“… a very good alternative to flow cytometer …”
“This is so convenient and easy to use.”
Cellometer K2 Cell Counter
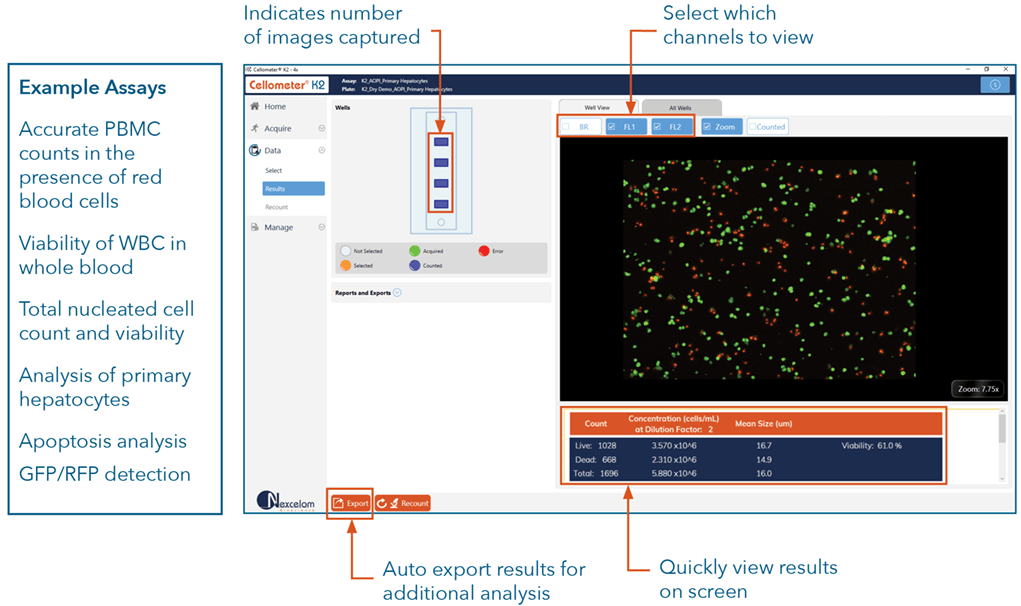
Our most popular cell counter, the Cellometer K2, powered by Matrix software, utilizes brightfield imaging and dual-fluorescence imaging to quickly and accurately identify and count individual cells. Cell count, concentration, diameter, and % viability are automatically calculated and reported.
The Cellometer K2 provides:
- Dual fluorescence and brightfield imaging – stain only nucleated cells for the
most accurate count and viability information - Fast results – count, size, concentration, and viability calculations in <60 seconds
- Analysis of complex samples – designed for analysis of complex and messy samples
including whole blood, peripheral blood, cord blood, and bone marrow - 21 CFR Part 11 ready – optional add-on that includes an audit trail, user access
control, and digital signature - Multiple fields of view – increased accuracy with the ability to capture one, four, or
eight images per sample - Built-in predefined assays – quickly analyze viability, apoptosis, and transfection efficiency
- Built-in cell types – includes saved parameters for over 400 cell types
- Small sample volume – only 10 µl of cell sample required
- Customizable reports – includes predefined reports with the ability to create new ones
with graphs, images, charts, and tables - Multi-language support – over 7,000 languages available
“The Cellometer K2, coupled with ViaStain AOPI stain, allows users to easily stain nucleated cells in samples containing red blood cells. No lysis buffer is required, which makes getting results very quick.”
Simple and Fast Cell Counting Workflow with Results in Seconds

“We love our Cellometer K2 and every lab should have one! Gone are the days of manual cell counting and we can now reliably and quickly count thousands of cells in a few seconds.”
Accuracy from Cell Lines to Primary Samples
The Cellometer K2 can be customized to handle a variety of cell types, including primary cells, tumor digest, insect cells, cell lines, fragile cells, and more at low or high concentrations.
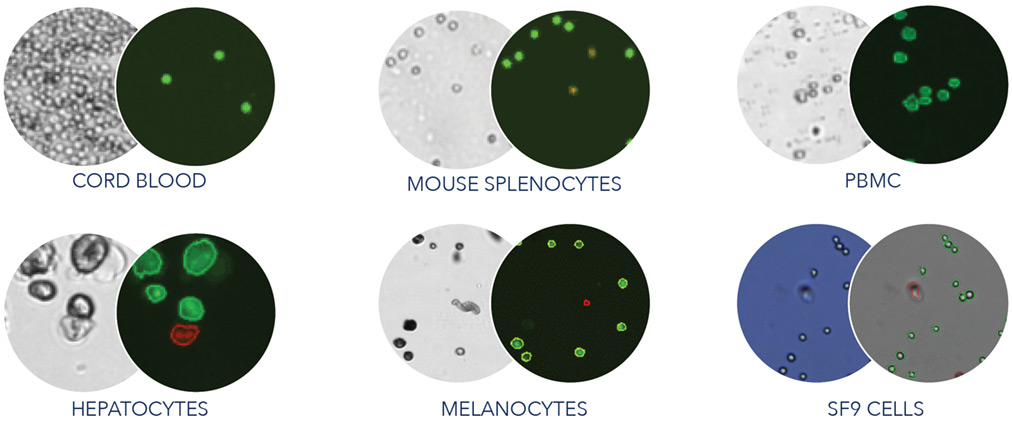
Predefined Assays and Cell Types
Take the guesswork out of setting up your cell quantification experiments. The Cellometer K2 comes with frequently used assays and cell types with predefined settings to ensure consistent results from sample to sample. Easily build custom assays and cell types to fit your experimental needs.
Not sure what settings to use? Our customer success team and field application specialists are here to help you develop fit-for-purpose assays and protocols for your specific research and development requirements.
“This unit works fast to get accurate and precise numbers. It takes a fraction of the time trypan blue or other manual methods for cell counting. It allows for greater throughput of samples and simplifies our workload. It is simple to use and miles ahead of any of the competitors. ”
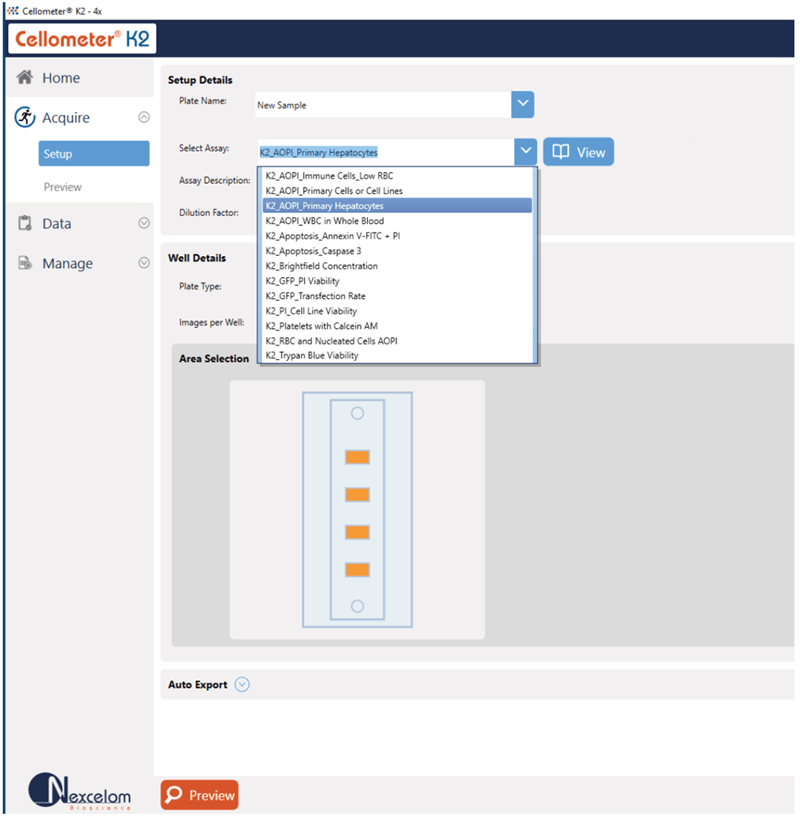
Capture Multiple Fields of View
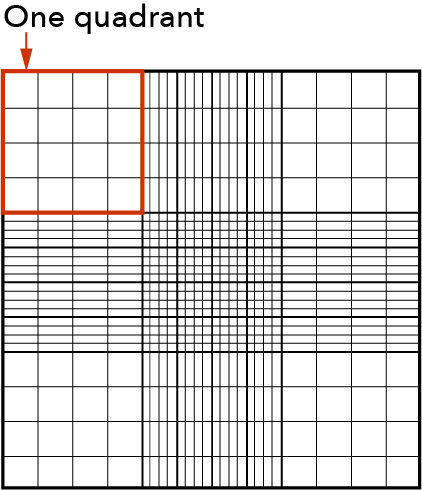
Capture one, four, or eight images per sample. The instrument default is set to four images, which is the equivalent of six quadrants on a hemocytometer. Eight images are equivalent to twelve quadrants on a hemocytometer. The ability to capture multiple images improves the cell counting dynamic range and accuracy of results.
“[Cellometer] K2 allows us for faster, reliable and more accurate cell counts that our lab uses so frequently. The process itself is simple and intuitive compared to the other machine we used to have. Such a great improvement!”
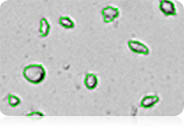
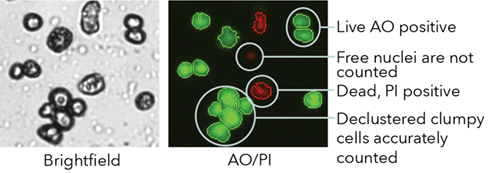
Dual-fluorescent Staining for Clumpy Cells
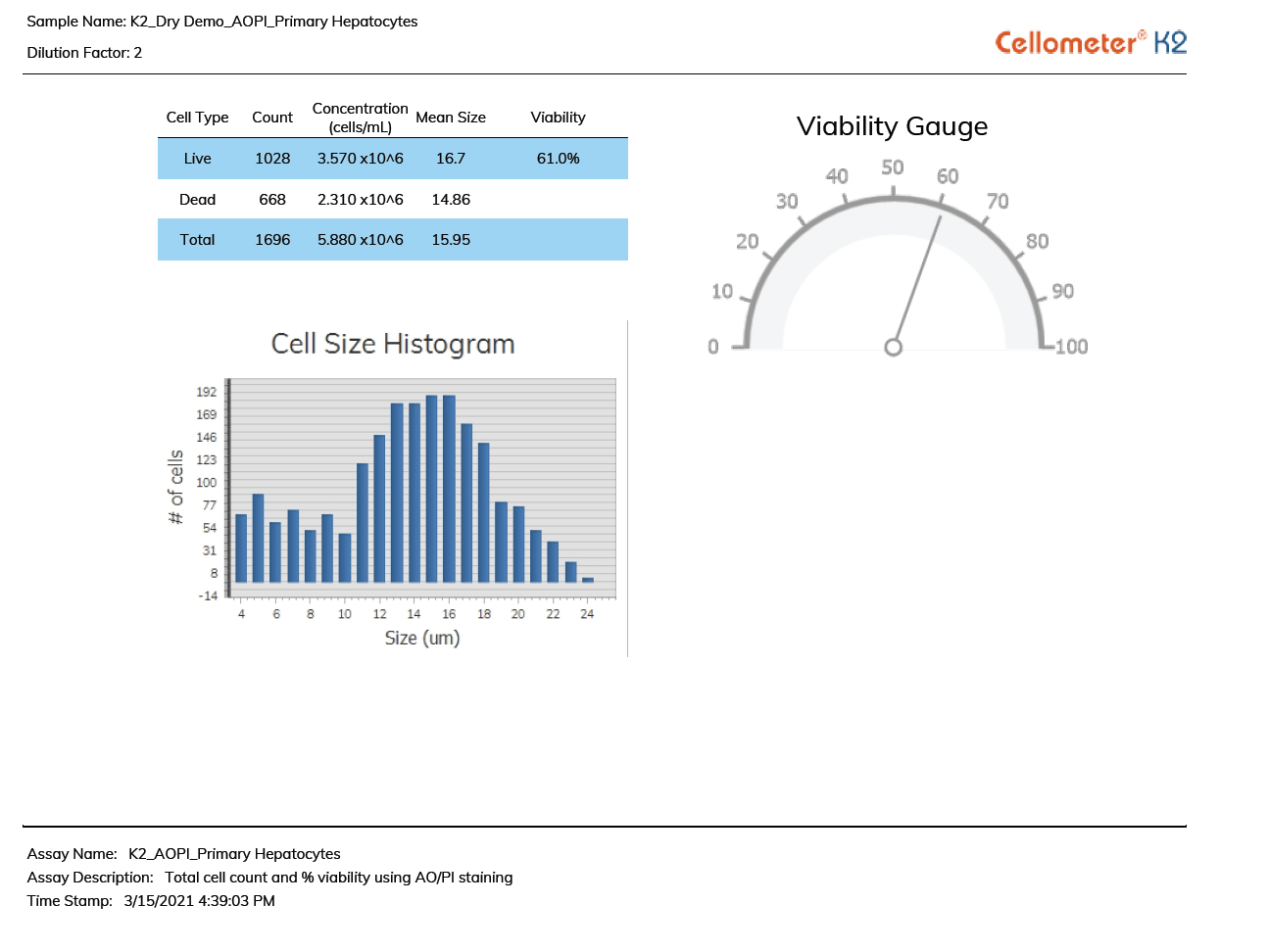
The fluorescent image (far right) shows bright green AO-positive hepatocytes declustered by the Cellometer K2 algorithm. Red circled hepatocytes are PI-positive (dead) while free nuclei are not counted.
Predefined and Customizable Reports
Use default or customized reports based on the data and images you want to be included for your specific experimental needs. Automatically export images and data reports including CSV, Excel, Word, or PDF files. Perform statistical analysis for a wide range of parameters such as average, variance, min/max, and standard deviation of cell size.
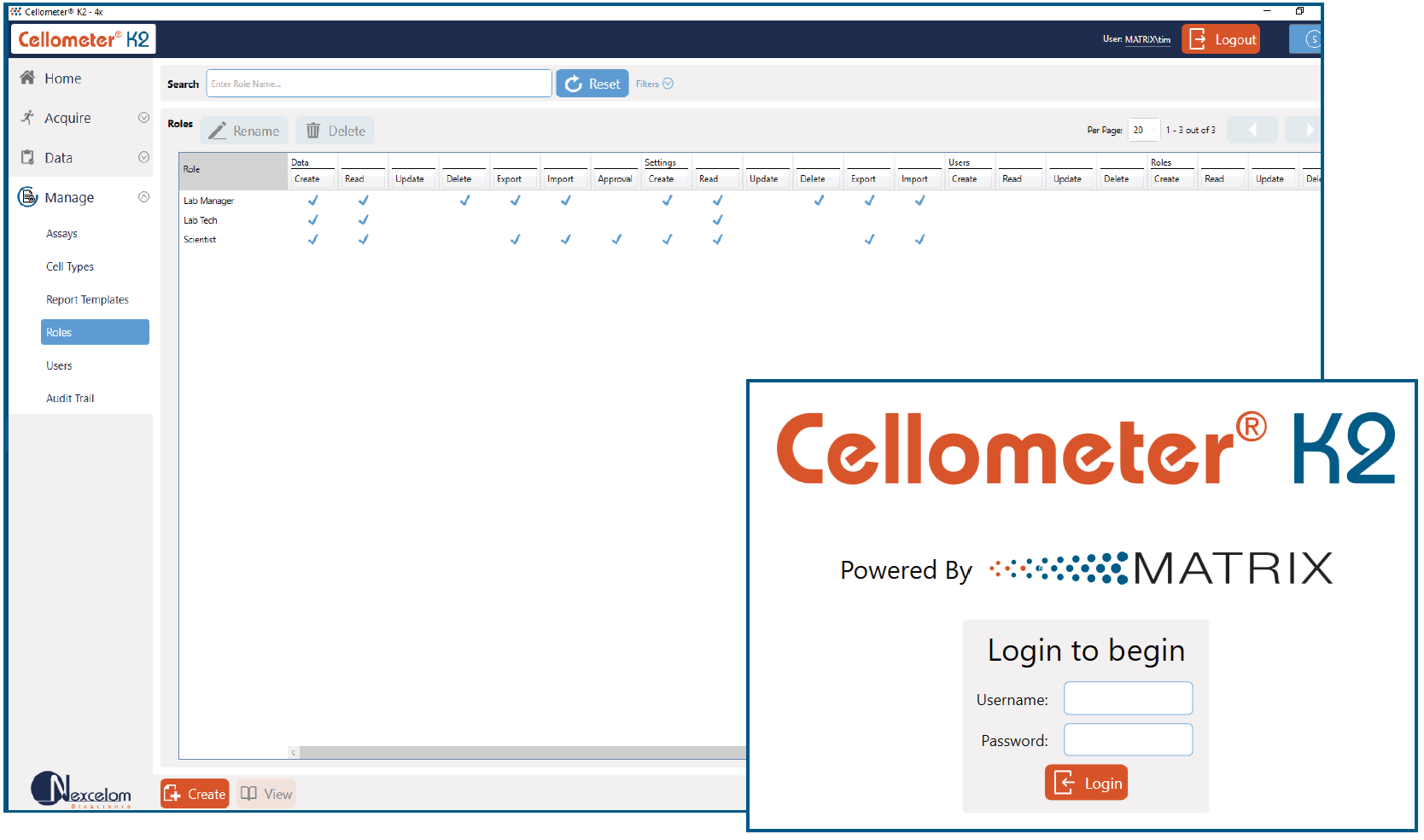
21 CFR Part 11 Ready
An optional module can be purchased to meet the requirements for 21 CFR Part 11. The additional module comes with the following features:
- User login with passwords
- User assigned permissions
- Audit trail
- Error log files
- Electronic signatures
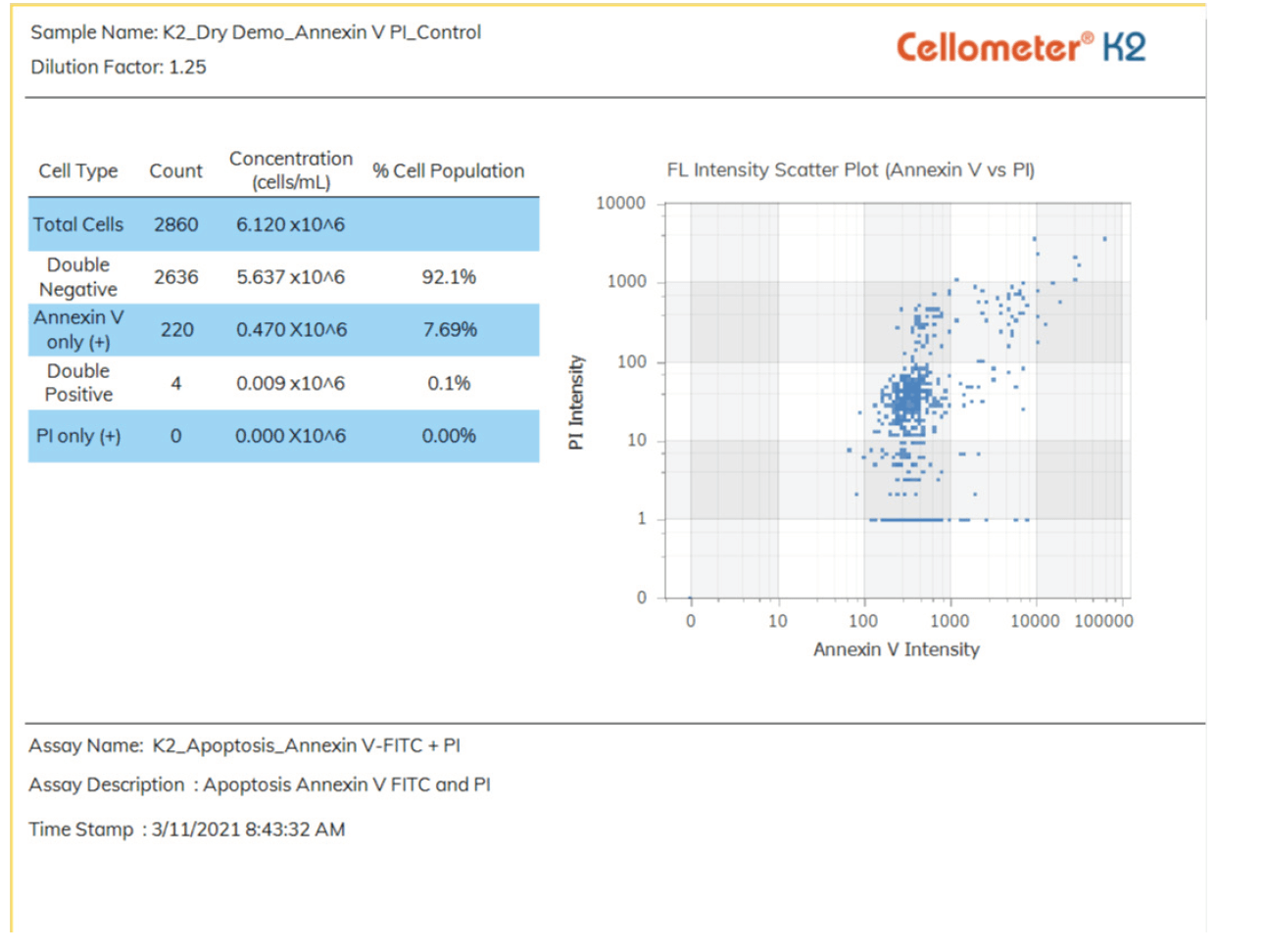
Live/Dead Nucleated Cell Counts using Dual-fluorescence
Why Dual-fluorescence?
Because brightfield cell counting does not differentiate nucleated from non-nucleated cells and trypan blue staining is not as easy to detect as fluorescent staining, dual-color fluorescence is strongly recommended for accurate viability analysis for primary cells. The K2 is equipped with standard assays for dual-fluorescence analysis of a variety of cells stained with Acridine Orange and Propidium Iodide (AO/PI).
The AO/PI Method
Acridine Orange (AO) is a nuclear staining (nucleic acid binding) dye permeable to both live and dead cells. It stains all nucleated cells to generate green fluorescence. Propidium Iodide (PI) can only enter dead cells with compromised membranes. It stains all dead nucleated cells to generate red fluorescence. Cells stained with both AO and PI fluoresce red due to quenching, so all live nucleated cells fluoresce green and all dead nucleated cells fluoresce red.
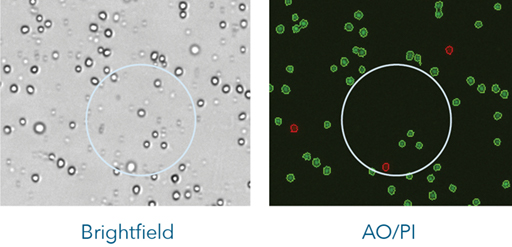
The brightfield image on the left shows the combination of nucleated cells, red blood cells, and platelets present in the sample. The red blood cells are not visible in the fluorescent image on the right, only the live (green) and dead (red) nucleated cells are counted.
No Interference from Red Blood Cells, Platelets, or Debris
The dual-fluorescence AO/PI method utilizes nuclear staining dyes that bind to nucleic acids in the cell nucleus. Because most mature mammalian red blood cells do not contain nuclei, only live and dead mononuclear cells produce a fluorescent signal. There is no need to lyse red blood cells, saving time and eliminating an extra sample preparation step. Red blood cells, platelets, and debris are not counted in the fluorescent channels.
Analysis of Clumpy and Irregular-shaped Cells
Including NCI-60 and clumpy MCF-7 Cells
NCI-60 is a group of 59 human cancer cell lines (originally 60) developed by the National Cancer Institute for screening purposes.
- 57% of the NCI-60 cell lines are clumpy, contain debris, or display large variations in cell shape or size
- All 59 NCI-60 cell lines have been successfully validated on the Cellometer Image Cytometer
All 40 of the NCI Comprehensive Cancer Centers use Cellometer Cell Counters.
Clumpy Cells
The MCF-7 breast cancer cell line can be very clumpy. The Cellometer pattern-recognition software identifies and counts individual cells within these cell clumps for accurate analysis (shown above).
Irregular-shaped Cells
The Cellometer cell roundness setting can be adjusted for recognition and counting of irregular-shaped cells, such as RD cells and activated T-cells.
10x Faster than Manual Counting
Counting 1 x 106 cells takes approximately 5 minutes with a manual hemacytometer. Counting live and dead cells sometimes takes twice as long. The Cellometer K2 Image Cytometer calculates cell count and concentration for live and dead cells and % viability in just 60 seconds.
Improve Data Accuracy & Consistency
- Eliminate wash steps
- Eliminate judgment errors
- Eliminate interference from RBCs
- Eliminate recording & calculation errors
- Reduce counting time … run more experiments
Imaging / Counting Chambers: No Washing or Contamination
We offer two different disposable counting slides, one with protective coverings on both sides and a ready-to-use option packed in microscope slide boxes.
Several key advantages include:
- No clogging
- Time savings — no washing
- No risk of cross-contamination
- Reduce biohazard risk to users
Applications for Cellometer K2 Fluorescent Viability Cell Counter
Peripheral Blood Mononuclear Cells (PBMC)
Measure live cell concentration and viability without lysing red blood cells for consistent results from patient samples. »
Measure GFP Transfection
Rapidly identify fluorescence-positive cells from a sample, calculate cell concentration, size, and determine the GFP transfection percentage automatically. »
WBCs in Whole Blood
Measure nucleated cell concentration without lysing red blood cells using nuclear staining dyes (AO), for human and mouse blood. »
Immunology Research
Quantify cell viability and concentration for a variety of immunologically relevant samples such as: bone marrow, cord blood, splenocytes, lymphocytes, isolated mononuclear cells, tumor digests, murine samples, and others. »
Fresh & Cryo Preserved Primary Hepatocytes
Measure live hepatocyte concentration and viability from fresh and cryo preserved samples using dual-fluorescent nuclear stains for human, rat, mouse, and horse. »
NCI-60 Cancer Cell Lines
Measure live cell concentration and viability of cancer cell lines used in oncology research and biology research. »
Adoptive Cell Transfer Therapy
Perform cell based assays and measure cell size, viability, and concentration of cell lines and primary samples used in adoptive cell therapy research. »
Cell Viability Measurement Using Trypan Blue or AO/PI
When should you use trypan blue and when should you use acridine orange/propidium iodide to measure cell viability? »
Cell Viability and Necrosis
Cell viability is performed using various fluorescent membrane exclusion dyes, such as PI, EB, 7AAD, and others. This assay is performed by enumerating cells in captured bright-field and fluorescent images. And Necrotic cells are detected using propidium iodide. »
TNC Concentration & Viability for Clinical (Blood) Samples
Analyze fresh and processed blood and bone marrow samples without lysing: no interference from RBCs. »
Cell Size Assay
Performing cell size measurement assay and using cell size to count cells within preset cell size parameters. For adipocytes, stem cells, Sf9 cells, dendritic cells, and others. »
Cellometer K2 Specifications
| Includes |
|
| Available Accessories |
|
| Imaging Performance | Cell Size: 4-90 microns Conc. Range: 105 – 107 cells/ml Brightfield imaging, fluorescent imaging and pattern-recognition software to quickly and accurately decluster, identify and count individual cells. |
| Instrument Specifications | Weight: 23.0 lbs. (10.4 kg) Dimensions: Width: 6.0” (15.2 cm), Depth: 8.5” (21.6 cm), Height: 14.0” (35.6 cm) Input to Power Adapter: 100-240 VAC, 50/60 Hz, 1.0A Output to Instrument: 12 VDC, 3.34A |
| PC / Laptop Minimum Requirements: (If purchasing Cellometer without PC laptop) |
|
| Available Fluorescence Optics Modules | Excitation / Emission: 470nm/535nm Example Fluorophores:
Excitation / Emission: 540nm/660nm
|
Cellometer K2 Resources
Manuals
- Cellometer K2 User Manual
- Cellometer K2 Quick Start Guide
- Cellometer K2 with Matrix User Manual
- Cellometer K2 with Matrix Quick Start Guide
- Matrix Software User Manual
Application Notes
Product Literature
- Cellometer K2 Cell Counter Brochure
- Cellometer K2 Flyer
- Cellometer K2 Spec Sheet
- Cellometer K2 Single Cell Sequencing
Blog Posts and Literature Reviews
- Maximize Output from Precious Samples for Single Cell Genomics Platforms
- Cellometer Fluorescent Cell Counters for Mouse Samples
- Streamlining single-cell sequencing workflows using Cellometer and Cellaca high-throughput cell counters
Videos
- How to Improve your Primary Cell Analysis: Make it Accurate, Quick and Simple
- How to Perform High Throughput Cell Cycle Assays
Training Videos
Cellometer X2 and K2 Automated Cell Counter Demo
Can’t watch YouTube? See video here
Customer Testimonials
Worth the investment
This instrument [Cellometer K2] is definitely worth the investment. It has a plethora of uses and now serves as the go-to instrument for not only counting cells but for measuring the titer of baculovirus-infected insect cells. Additionally, the customer service representatives are very helpful.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Worth the investment
This instrument [Cellometer K2] is definitely worth the investment. It has a plethora of uses and now serves as the go-to instrument for not only counting cells but for measuring the titer of baculovirus-infected insect cells. Additionally, the customer service representatives are very helpful.
Consistent and dependable
We use Cellometer K2 for cell counting. This is so convenient and easy to use. Each slide can be used for two different cell lines and the results show the total number of cells, the number of live cells and dead cells. So we can use this for a lot of experiments which are based on cell numbers and make our results more consistent and dependable.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Shan Lu
Consistent and dependable
We use Cellometer K2 for cell counting. This is so convenient and easy to use. Each slide can be used for two different cell lines and the results show the total number of cells, the number of live cells and dead cells. So we can use this for a lot of experiments which are based on cell numbers and make our results more consistent and dependable.
Shan Lu
Reproducible results
In comparison to the standard method for manual cell counting, Cellometer K2 helps me in counting cells efficiently and quickly with reproducible results. I use it mainly for assessing cell vitality by trypan blue exclusion assay.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'This machine has transformed the way I culture
This machine [Cellometer K2] has transformed the way I culture. No longer do I have to spend hours out of my day to manually could cells. It has changed the way I see cell culture from a menial chore to something I look forward to every day. Cheers NEXCELOM for a job well done!!!!
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'This machine has transformed the way I culture
This machine [Cellometer K2] has transformed the way I culture. No longer do I have to spend hours out of my day to manually could cells. It has changed the way I see cell culture from a menial chore to something I look forward to every day. Cheers NEXCELOM for a job well done!!!!
Allowed us to enter a new field of cell culture
The K2 has allowed us to enter a new field of cell culture with best practices already in place. Not having to optimize the use of a cell counter will save us a lot of time.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Zachary Schools
Very good alternative to flow cytometer
Cellometer K2 is very compact, reliable and easy to use. Our lab uses this counter to count live and dead cells for our experiments. It is a very good alternative to flow cytometer for cell cycle and apoptosis studies.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Easy to use
Cellometer [K2] is easy to use. I like that the program can calculate transfection efficiency, one of the things that we do on a normal basis in our lab but we were not able to quantify in the past.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'This has made our life much easier.
We really like this instrument [Cellometer K2]. We were counting cells manually until now. This has made our life much easier.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Quicker and more accurate
Cell counting is not only quicker and more accurate [with Cellometer K2] but assays that previously required a flow cytometer, such as Annexin V/PI apoptosis analysis, can now be completed in our lab!
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Fancy and compact
The design of the instrument is very fancy and compact. It can have its own screen or you can use a separate screen. Initially, our lab was using the manual method of cell counting by microscope. But [Cellometer] K2 from Nexcelom has made our life easy and provided reproducible counting.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Fancy and compact
The design of the instrument is very fancy and compact. It can have its own screen or you can use a separate screen. Initially, our lab was using the manual method of cell counting by microscope. But [Cellometer] K2 from Nexcelom has made our life easy and provided reproducible counting.
Reliable machine
The instrument [Cellometer K2] is being used in our lab for counting different types of immune cells, mostly neutrophils (but enucleated cells as well). It has been a reliable machine, no problems in general.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Convenience and speed
The convenience and speed of our Nexcelom K2 and Vision Cellometers allowed us to dramatically scale up our processing of mononuclear cells. Our sales and technical representatives have always been helpful and responsive to any inquiries.
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Stephanie Sieja
Simple and intuitive
[Cellometer] K2 allows us for a faster, reliable and more accurate cell counts that our lab uses so frequently. The process itself is simple and intuitive compared to the other machine we used to have. Such a great improvement!
function positionLinkBlock(targetContainer) { if (targetContainer != null) { var strLinkBlock = 'Hanh Chi Do
Customer Publications using Cellometer K2
Our customers include: