Ongoing Challenges in Immunotherapy Development Against Metastatic Disease
Metastatic spreading from solid tumors continues to be one of the greatest challenges facing oncologists and clinicians, often leading to a fatal outcome for many patients. Lung, liver, and bone are common sites for tumor metastases, making the design of novel therapeutics to destroy these disseminated tumor cells a priority for researchers (1).
Adoptive immunotherapies could be a promising way to treat several types of solid tumors (2). Chimeric antigen receptors (CAR), consisting of the antigen-binding region of a monoclonal antibody linked with an intracellular CD3 signaling domain through a T cell receptor transmembrane domain, are engineered onto the patient’s T-cells; in effect “training” the patient’s immune system to attack and eliminate cancer (3). To date, these therapies approved by the FDA are used to treat hematologic malignancies and have demonstrated high efficacy in the clinic (4,5).
Unfortunately, these CAR T-cell therapies are patient-specific, have complex manufacturing workflows, and come with the risk of severe side effects, including cytokine release syndrome and other neurologic and immunologic adverse effects (6). For these reasons, the development of new therapies using alternative CAR effector cells is a priority.
Flexible Antigen Targeting Using NK Cells
Natural Killer (NK) cells as an alternative to T-cells is an attractive option due to their relative safety in an allogeneic “on-demand” format and potent anti-tumor activity. Challenges remain in the adoption of this technology for the targeting of solid tumors, with antigen escape remaining a key barrier as solid tumors have very diverse surface antigen repertoires (7).
In the present study, Grote et al at the Children’s hospital in Tübingen conducted several preclinical experiments utilizing a molecular adapter CAR (AdCAR) platform that redirects CAR recognition to a linker epitope, biotin-conjugated with an adapter molecule (AM), instead of target tumor antigens directly. Therapeutic antibodies against target antigens can be conjugated with biotin-AM easily, thus opening up new possibilities in quickly redirecting the specificity of these treatments without having to re-engineer the entire CAR.
Imaging cytometry as a useful tool to access AdCAR-mediated cytotoxicity in a 3D tumor cell model
The Celigo Imaging Cytometer is a robust, high-throughput imaging platform that quickly delivers quantitative data from brightfield and fluorescent images. The system quickly scans the entire surface area of microwell plates while advanced analysis algorithms in the software segment the objects present to determine the number, size, fluorescent intensity, and other variables (Figure 1).
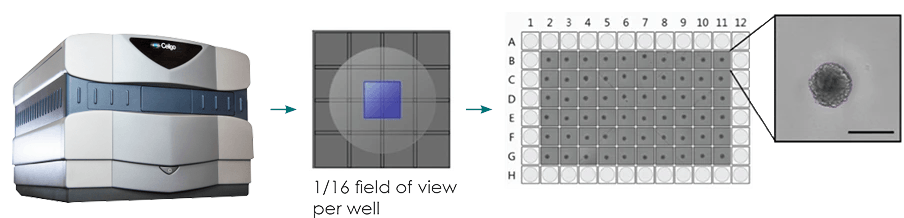
Figure 1: Typical imaging workflow for brightfield imaging of 3D spheroids. Centrally located spheroids can be imaged in brightfield using sub-sampling of the well area (only imaging the center), which decreases the overall imaging and analysis time to less than one minute per plate.
Grote et al utilized the Celigo to image 3D tumor spheroids, which are employed by researchers looking for a model more representative of the in-vivo situation. Spheroids were grown in culture for four days then co-incubated with either the AdCAR-transduced or parental NK-cells, in the presence or absence of biotinylated antibodies. NK-mediated cell lysis was assessed by measuring the integrated fluorescence intensity of the spheroids treated with NK cells and/or antibodies and correlating those levels with those of the control, untreated spheroids. Some antibodies were effective in reducing the spheroid sizes and fluorescent intensity (Figure 2).
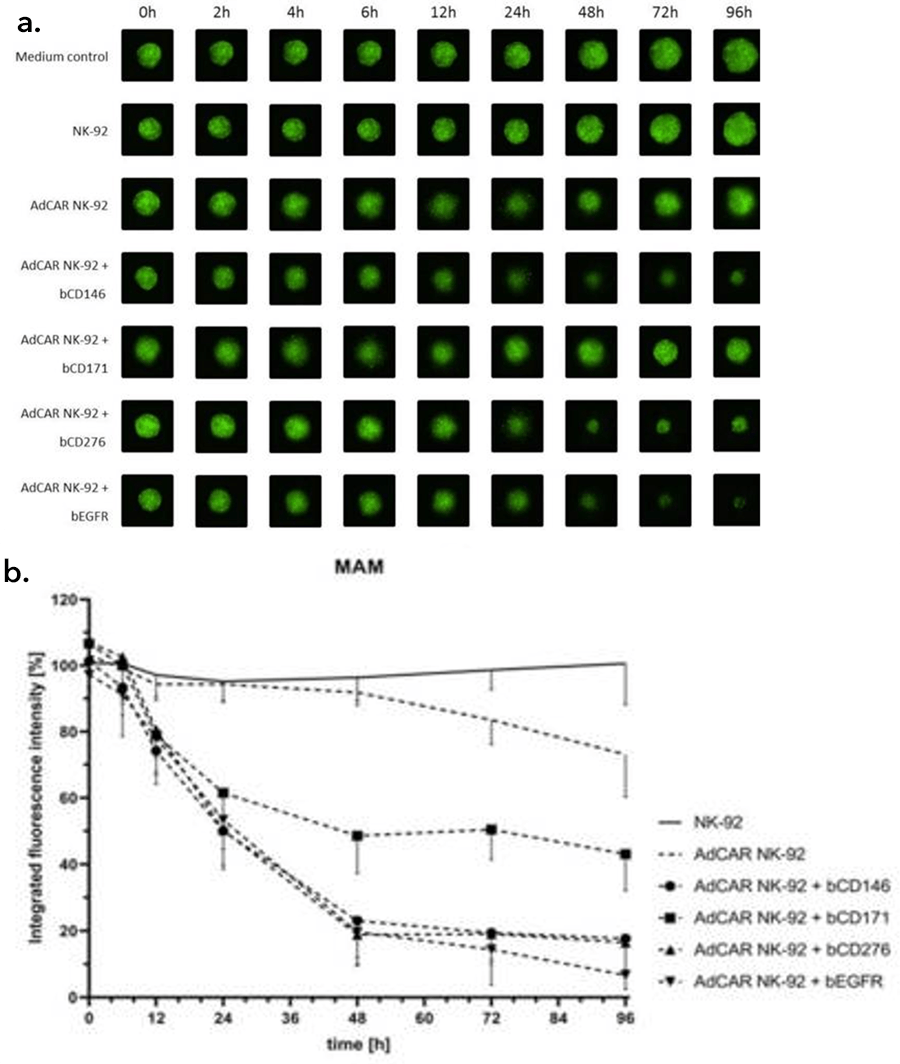
Figure 2: NK-cell mediated lysis of 3D tumor spheroids. a.) A GFP-transduced cell line was grown as 3D spheroids in ultra-low attachment plates and then co-incubated with the AdCAR or parental NK-cells with or without the indicated biotinylated antibodies for 96 hours. Images show representative spheroids at indicated timepoints, b.) Integrated fluorescence intensity as measured by the Celigo Imaging Cytometer, compared to untreated control spheroids, shown as mean SD, n = 3. Figure and text adapted from Grote et al, 2021.
Quantitative, High-Throughput 3D assays with Celigo.
Other types of 3D assays are possible with Celigo. The instrument can also be used to assess the health of the spheroids by utilizing viability dyes such as Calcein AM and PI with apoptosis markers. Together, these will indicate if a treatment is cytotoxic and kills the tumorsphere or if it is successful at stopping cell growth.
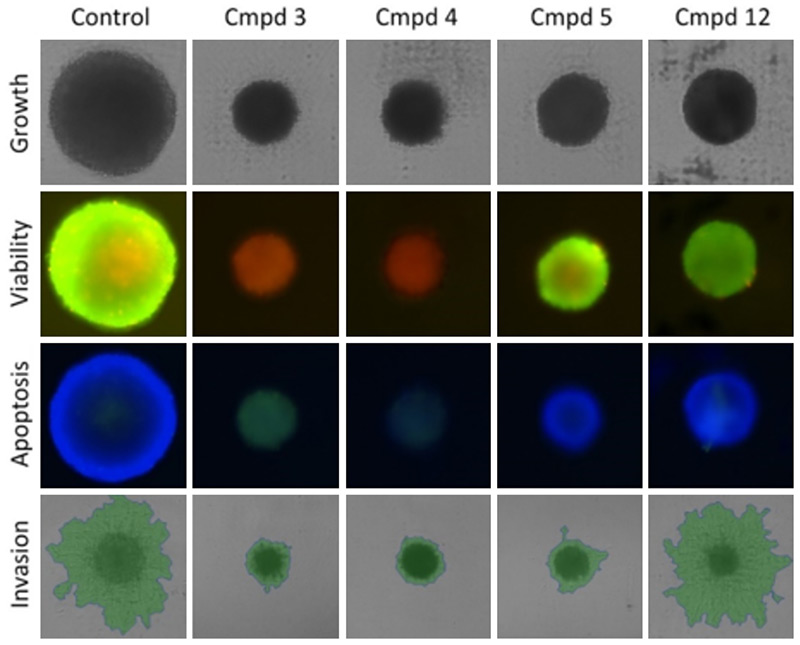
Figure 3: Possible 3D assays with Celigo. The effect of various compound treatments is quantified in various ways. The top row shows brightfield imaging for growth/size determination. Viability is assessed with the ratio of Calcein AM over PI intensity, with higher green fluorescence indicating higher viability. Apoptosis is assessed with the green signal from our caspase 3 reagent, with blue from the DAPI as a counterstain. Finally, invasion into matrigel can be measured by brightfield imaging and segmentation (green overlay).
The Celigo whole-well image cytometer for adherent and suspension cells is an excellent tool to help with 3D assays. Implementing the Celigo Image Cytometer opens the avenue for a more in-depth analysis that is both high-throughput and automation-ready. Learn more about 3D phenotypic cellular models and the direct cell counting assays for Immunotherapy and Virology on our website or contact us directly to book a seminar or live demonstration today!






Leave A Comment