Solid tumor biology has become a critical step in accessing the potency of many cancer drugs. 3D spheroids and organoids can provide measurements such as size differences under drug conditions or metabolism and diffusion rates critical to drug development. The limitation has been in acquiring and analyzing 3D structural data in an efficient and high-throughput manner. Many studies rely on endpoint analysis of digested tumors as a read-out for drug response. Makundan et. al. were in search of a method to screen various drug candidates against multiple solid tumors in a high-throughput manner with minimal cell handling. They relied on Nexcelom Bioscience and the Celigo Image Cytometer to accomplish this.
Individually track thousands of spheroids
Makundan et. al. collaborated with researchers from Nexcelom Bioscience to establish a semi-quantitative method to image and analyze thousands of spheroids at once within a single well. They used the Aggrewell 400 microwell plates to seed and grow approximately 1200 spheroids within each well and then used the Celigo Image Cytometer to image and analyze each spheroid simultaneously (Fig 1). Spheroids were stained with Calcein AM (CAM) and Propidium Iodide (PI) to measure viability under different drug candidates at various concentrations. Many of these candidates showed promising decreases in CAM and increases in PI with rising dosage (Fig 2). In addition, there was also a correlation between spheroid size and drug concentration after seven days. Many of the conditions that led to changes in viability also caused a drop in overall spheroid size, indicative of tumor shrinkage (Fig 2).
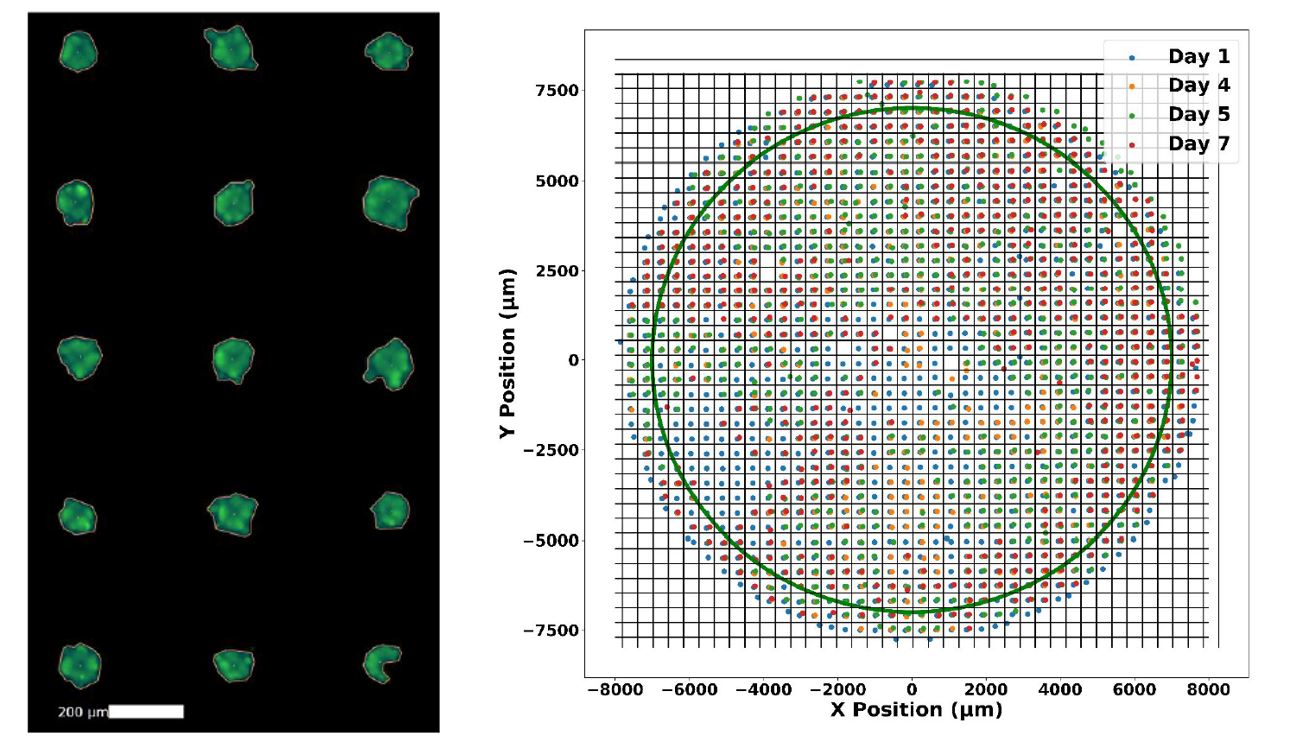
Figure 1. Images captured on the Celigo Image Cytometer showing individual spheroid analysis (left) and overall tracking of thousands of spheroids within a given well (right).
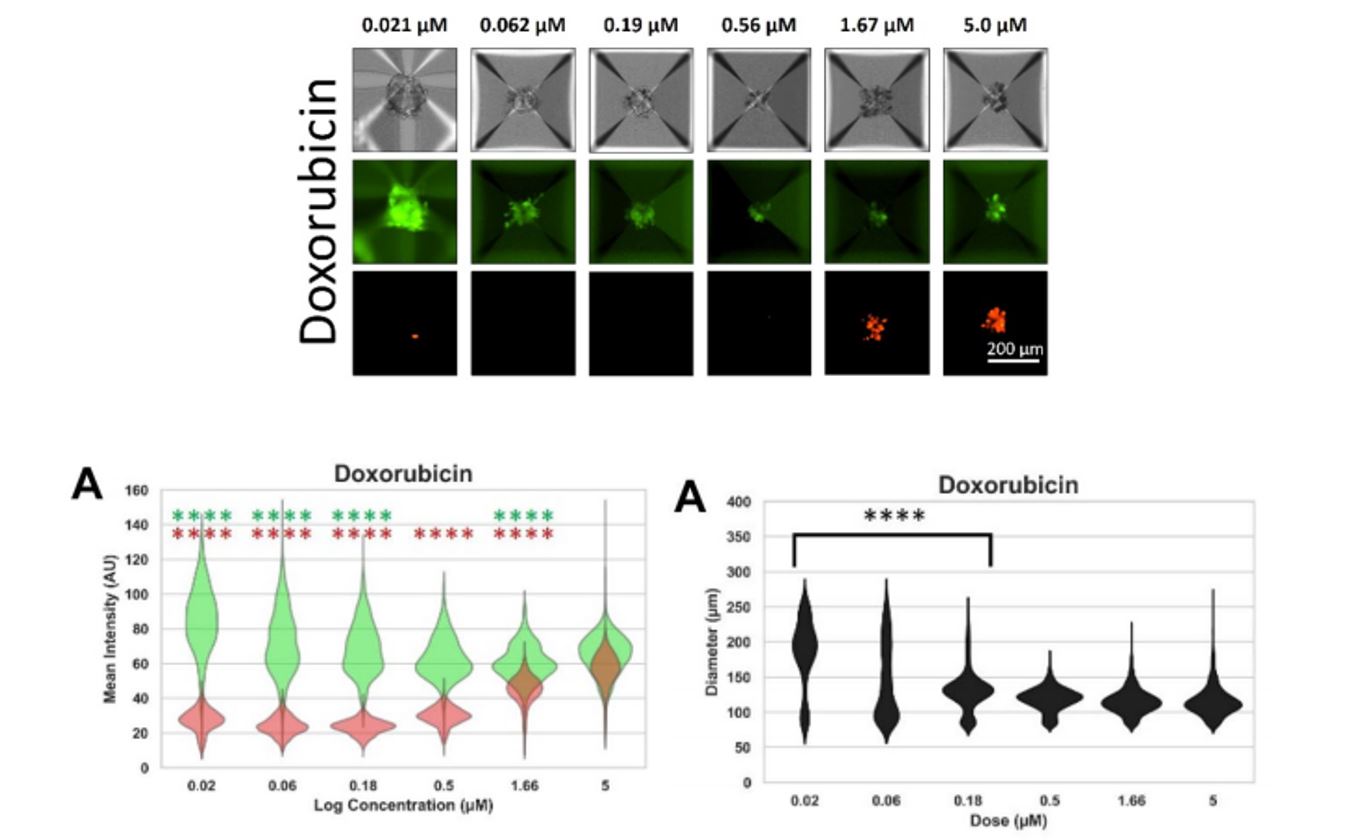
Figure 2. Viability and size measurements for one cancer drug used in this study. CalceinAM and Propidium Iodide fluorescent intensity were imaged and measured over increasing dosage of Doxorubicin (top and bottom left). The spheroid diameter was measured under different dosage conditions (bottom right).
As a comparison, CellTiter96 (MTS) was used as an orthogonal assay for the viability of post-treated spheroids. The results showed similar trends of decreases in viability that were observed with changes in CAM and PI. Also, Makundan et. al. observed a dose-dependent drop in spheroid size that correlated very well with the CellTiter96 viability results (Fig 3). This supports the ability to use tumor shrinkage as a screening tool for new therapeutic approaches.
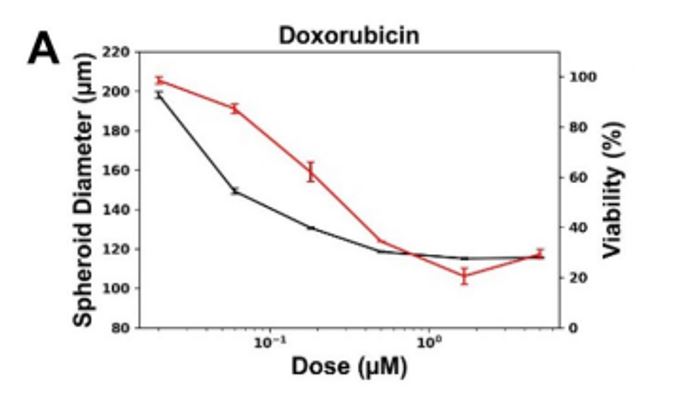
Figure 3. Correlation of spheroid size and CellTiter96 viability measurement.
In conclusion, Makundan et. al. designed an approach to testing various drug compounds on a large number of spheroids simultaneously. By employing the use of the Celigo Image Cytometer they were also able to image and analyze all these spheroids independently in a matter of minutes for large-scale statistical data points. This approach allowed for a high-throughput analysis of individual spheroids that could give insight into potential subpopulations that would not be easily detected in bulk studies. In addition, for patient-derived tumor samples with heterogeneity levels, this could be a great tool to quickly identify various conditions that may be beneficial in attacking these diverse samples. Nexcelom Bioscience and the Celigo Image Cytometer are excellent resources for researchers that are looking to speed up their workflows and design new approaches to scientific questions. The Celigo Image Cytometer offers a wide variety of applications for automated cell counting and cell-based assays with intuitive software analysis tools and automation options to accommodate high-throughput needs. Learn more about cellular models and direct cell counting assays on our Celigo Image Cytometer Applications page or contact us directly to book a seminar or live demonstration today.
References
Mukundan, S., Bell, J., Teryek, M., Hernandez, C., Love, A. C., Parekkadan, B., & Chan, L. L. (2022). Automated Assessment of Cancer Drug Efficacy On Breast Tumor Spheroids in Aggrewell™400 Plates Using Image Cytometry. Journal of fluorescence, 32(2), 521–531. https://doi.org/10.1007/s10895-021-02881-3






Leave A Comment