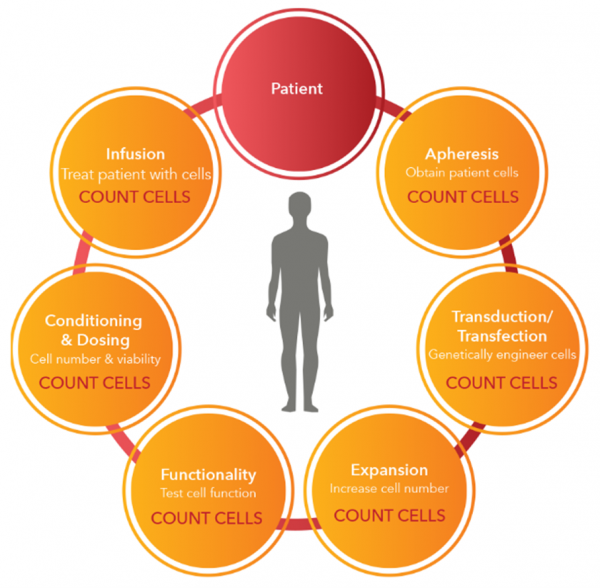
Cell-based treatments are life-saving therapies with the potential to offer cures for cancers and other diseases. Autologous and allogeneic cell therapies start with the isolation of immune cells from either a patient or from healthy donor sample blood followed by the activation, expansion, and ex vivo genetic modification of selected immune cells. The processes to effectively develop and manufacture safe, high-efficacy cellular therapeutic products are complex and costly. As the demand for these promising therapies increases, the focus is now on how to make them more readily available in a faster and more cost-effective manner.
T cell immunotherapy workflows start with a cell isolation step, followed by T cell activation, and cell expansion surveyed by extensive cell characterization. In both basic research and translational medicine, there is a need for high-quality, reliable, well-characterized reagents covering the complete workflow. BioLegend supports CAR-T manufacturing processes through the development of a reagent for FBS and hAB serum replacement in T cell culture. This less costly substitute represents a major step forward in developing reproducible and controlled studies, able to generate safe, well-defined cell products.
To ensure the safety and efficacy of the “living drug”, all materials used in the manufacturing process are required to have high quality and controllable batch-to-batch variation based on FDA guidelines (Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs)- 2020 FDA). In addition, robust analytical methods are needed to routinely monitor immune cells’ purity, identity, and quantity throughout the whole process.

With the replacement of FBS and human-derived sera in T cell culture, the reproducibility of the cell culture can be better controlled to support the manufacture of defined cell products. Learn more about these improvements in this webinar, where BioLegend’s VP of Product Development, Dr. Jessie Ni, describes the development and validation of “A novel xeno-free serum substitute for T and NK cell culture to support clinical-grade manufacturing”.

The second webinar speaker, Dr. Sergio Mojica of Nexcelom, now part of Revvity, highlights innovative imaging cytometry tools for accurately counting cells and performing in vitro immune cell functional analysis with reliable, robust, trackable, and easy workflows to ensure the manufacture of high-quality cell therapy products.
Watch our webinar on demand to learn more about:
- Development and validation of a reagent to replace FBS and hAB serum for better reproducibility, safety, and productivity in GMP manufacture of T cells and NK cells
- Image cytometry and its major applications in the development and manufacture of cell and gene therapies
- Application of high-speed and high-throughput image-based cytometry systems to reliably count and analyze immune cells in product development and manufacturing processes






Leave A Comment