Download our Poster
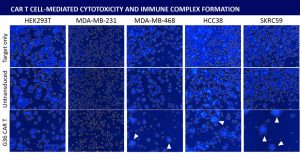
Abstract: Cancer immunotherapy has been gaining momentum in the field of cancer research. Specifically, Chimeric Antigen Receptor (CAR) T cell technology have introduced new methods to combat cancer. Direct cell-mediated cytotoxicity assays are required to assess the killing capability of the engineered CAR T cells. Traditionally, these assays are conducted by measuring the amount of released Chromium, calcein AM, or LDH molecules after the target cancer cells are killed with CAR T cells. These methods require a large amount of target cells which may not be ideal when working with donor primary samples. Additionally, they cannot specifically analyze the immune complexes formed during CAR T cell killing. Immune complex formation is a known event that may reveal information for effector cell migration. Recent advancement in imaging technologies have developed novel methods for assessing these immune complexes. In this work, we demonstrated an immune complex analysis method using the Celigo Image Cytometer to determine CAR T cell activity during co-culture with cancer cells. First, different target cancer cells (HEK293T, MDA-MB-231, MDA-MB-468, HCC38, and skrc59) are stained with ViaStainTM Tracer Blue dye, seeded in a 96-well plate, and incubated overnight. Next, untransduced T cells and G36-CAR T cells are added to the wells at 20:1 effector-to-target (E:T) ratios and co-cultured for 24 hours. Finally, the plate is scanned and the immune complexes were analyzed by confluence measurement in blue channel and compared to the untransduced T cells. Image cytometry analysis evaluated CAR T cell activities for all of tested target and effector cell combinations. Utilizing an image cytometry platform can visually confirm interactions between effector and target cells, thus making results highly accurate and robust. Unlike the traditional release assays, the ability to analyze the immune complexes formed during co-culture assays can provide additional important functional information of the effector cells, such as migration and clustering capabilities.






Leave A Comment