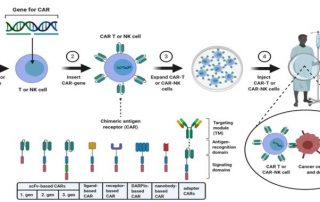
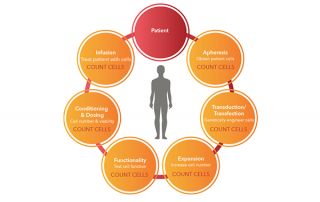
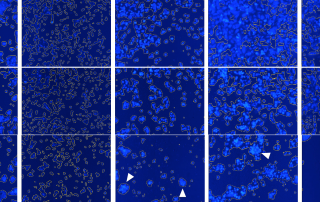
Download our Poster Abstract: Cancer immunotherapy has been gaining momentum in the field of cancer research. Specifically, Chimeric Antigen Receptor (CAR) T cell technology have introduced new methods to combat cancer. Direct cell-mediated cytotoxicity assays are required to assess the killing capability of the engineered CAR T cells. Traditionally, these assays are conducted by measuring the amount of released Chromium, calcein AM, or LDH molecules after the target cancer cells are killed with CAR T cells. These methods require a large amount of target cells which may not be ideal when working with donor primary samples. Additionally, they cannot [...]