Effective long-term cryopreservation of human mesenchymal stem cells is an essential component of regenerative medicine and tissue engineering applications.
The handling of stem cells is highly delicate and proper cold-chain storage represents a critical component to the transportation and distribution of these cellular therapies. “Off-the-shelf availability of human umbilical cord-derived mesenchymal stromal cells (UC-MSCs) for regenerative medicine application requires the development of non-toxic, safe, and efficient protocols for cryopreservation.”1
Comparisons of traditional cold-storage versus automatic cryopreservation
The process of placing cells into long-term cold storage requires repeated ambient exposures. The pendulum of fluctuating from extreme cold to room temperature has a great impact on cell activity and functionality. To fully compare different preservation processes, researchers used an automatic temperature-controlled cryopreservation system to reduce temperature shifts and investigate the contrast to manual cold storage methods.
UC-MSCs were employed to investigate the simulated manual process by being repeatedly transferred by a robotic arm between liquid nitrogen (−150 °C) and room temperature. UC-MSCs from the same batch were also processed with the automatic cryopreservation system, where the cells were maintained below-150 °C throughout.
Parameters measured to assess optimum conditions for UC-MSCs
Viability, percent recovery, adherence capability, cell proliferation, and multilineage differentiation ability were all used to compare the handling of UC-MSCs by the manual approach versus the automatic cryopreservation system.
UC-MSCs samples were stained with acridine orange (AO) and propidium iodide (PI) viability dye mixture to assess cell concentration, viability/survival, and percent recovery. The UC-MSCs were measured in triplicate per cell sample using the Cellometer Auto 2000 to determine the number of fold expansions.
After extensive transfers, the proliferation of UC-MSCs was compared between the traditional manual sample handling and the automatic system. Final cell recovery comparison results from Cellometer Auto 2000 numbers show the automatic system’s evasion of drastic temperature fluctuations retains proper proliferative capabilities.
Post-thaw UC-MSCs adherence capability
The Celigo Image Cytometer was used to acquire whole-well brightfield images and perform confluence analysis of the entire 96-well plate to determine the adherence capability of post-thawed UC-MSCs.
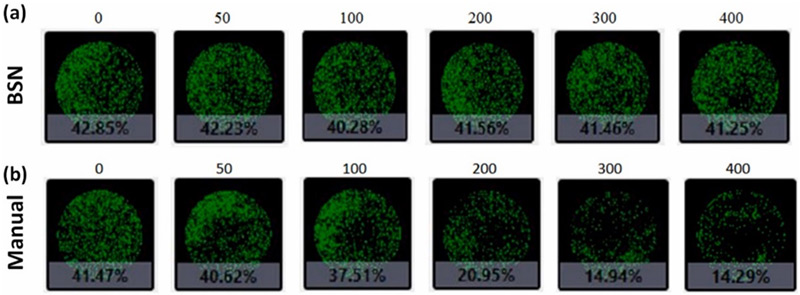
Whole-well images from a 96-well plate acquire by the Celigo Image Cytometer
The Celigo evaluated the adherence of UC-MSCs by assessing confluence at 0, 50, 100, 200, 300, and 400 cycles where the areas covered by the UC-MSCs are filled with a pseudo-green color. “As the cycle number increased, the confluence of UC-MSCs managed by the manual system decreased gradually from 41.47% to 14.29%, where a significant reduction began at 200 cycles. On the other hand, the confluence percentages of UC-MSCs managed by automatic system remained consistently at ∼42% for all cycle conditions.”1
The automatic cryopreservation system demonstrated higher viability, percent recovery, cell proliferation, and improved plate-adherence. This potential to optimize the entirety of cold-chain storage systems could “provide a powerful tool to develop safe, reliable, and efficient protocols for manufacturing and banking of UC-MSCs, improving their off-the-shelf availability for regenerative medicine applications.”1
Temperature regulated storage and transportation are prerequisite organizational steps within stem cell research, regenerative medicine, and tissue-related engineering. Understanding the mechanisms of cellular activities during temperature changes and characterizing the cells is necessary to find the optimal conditions imperative for proper long-term storage of cell therapies.
Learn more about how advanced technologies like the Cellometer Auto 2000 and Cellaca MX can help rapidly improve and optimize your cellular research.
Reference:
- Yanhong Xu, Leo Li-Ying Chan, et al., Optimization of UC-MSCs cold-chain storage by minimizing temperature fluctuations using an automatic cryopreservation system, Cryobiology, 2020, ISSN 0011-2240, https://doi.org/10.1016/j.cryobiol.2020.11.010. (http://www.sciencedirect.com/science/article/pii/S0011224020306544)






Leave A Comment