Drug repurposing, also known as drug repositioning is a powerful tool for the rapid expansion of available therapy options by identifying new therapeutic uses from pre-existing drugs. The SARS-CoV-2 pandemic continues to ravage the world causing untold medical and economic disruption. Novel vaccines and therapies are urgently needed for the treatment and prevention of new infections against this virus and to eventually restore normalcy. While social distancing and lockdown restrictions have helped to reduce infection rates in some areas, worldwide infection rates continue to climb, highlighting the urgent need for revolutionary infection control strategies.
SARS-CoV-2 is a novel virus and has no current established treatments. In partnership with government labs, biopharma companies around the world are committed to producing a vaccine and as of this writing, more than 180 are in development (1). Initiated by the US government, Operation Warp Speed has been aiming to have a vaccine approved by January 2021. Similarly, the World Health Organization has spearheaded a global initiative to fast track a Coronavirus vaccine by the end of 2021 (2). Creating a new vaccine against such a virus normally necessitates years of efficacy and safety trials.
Drug Repurposing against SARS-CoV-2
If a drug originally approved for another purpose is shown to have activity against SARS-CoV-2, it could be made available far more quickly than a brand-new drug. Interestingly, as the pharmacological, toxicity, and safety studies on pre-existing compounds have already been done, repurposing existing FDA-approved compounds may provide a faster way to arrive at new and effective treatments.
One study published early on in the pandemic by a large global team, cloned, tagged, and expressed 26 out of the 29 SARS-CoV-2 proteins in human cells to assess their binding affinity to human protein targets, identifying 332 high-confidence protein-protein interactions (3). Further investigation revealed 69 compounds that target 66 human proteins, a subset of which were assayed for antiviral activity.
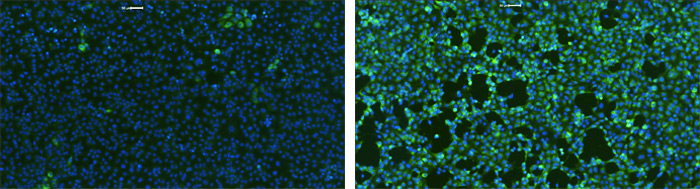
Example SARS-CoV-2 infected host cells labeled with DAPI and AF488 fluorescence to analyze the infection rate after treatments.
At this stage, the Celigo Imaging Cytometer from Nexcelom Bioscience provided a vital role in assessing the antiviral activity of the identified compounds. Human kidney cells were infected with SARS-CoV-2 and infection levels were assessed at different compound doses by antibody staining for the viral NP protein, while total cells were quantified with a DAPI counterstain. The proprietary F-Theta lens technology on the Celigo allowed the researchers to quickly image the entire surface area of microwell plates while the segmentation on the software helped to reliably identify infected vs. uninfected cell populations.
A similar approach was taken in a second study published in July 2020 (4). Using a similar method as the first study, the researchers profiled a large library of approximately 12,000 small molecules, either FDA-approved, in the clinical trial stage, or with extensive preclinical characterization. Of these, 21 were found to inhibit SARS-CoV-2 replication in a dose-dependent fashion, thirteen of which had effective concentrations that could be achievable within patients in a therapeutic setting. This demonstrates Celigo’s high-utility in screening thousands of compounds and rapidly assess infection levels of cells exposed to the virus
Drug Repurposing in Cancer Research
Similar to the aforementioned studies regarding the repurposing of existing drugs for use against SARS-CoV-2, the Celigo has been utilized for drug repurposing in the context of cancer treatment. In 2016, a group at the Danish Cancer Society Research Center screened 72 Cationic Amphiphilic Antihistamine (CAD) compounds, previously used to treat a variety of conditions, for anti-cancer activity against several non-small cell lung cancer lines (NSCLC) (5). The researchers performing this work used Propidium Iodide (PI), a non-membrane permeable cell death dye, to assess the efficacy of the compounds in killing the cancer cells. Subsequently, it was found that the use of these compounds is associated with a significant reduction in all-cause mortality among patients with non-localized NSCLC.
Other groups have conducted similar drug repurposing studies. In 2018, American and Chinese researchers screened over 1,000 FDA-approved compounds and found several that inhibit the proliferation of esophageal adenocarcinoma (6). In the same year, another study found 26 compounds with anti-tumor activity after screening 3,800 compounds (7). Both of these articles utilized experiments with Celigo to rapidly acquire images across many wells, and with Propidium Iodide as a marker for cell death, either in 2D monoculture or 3D tumor spheroid models.
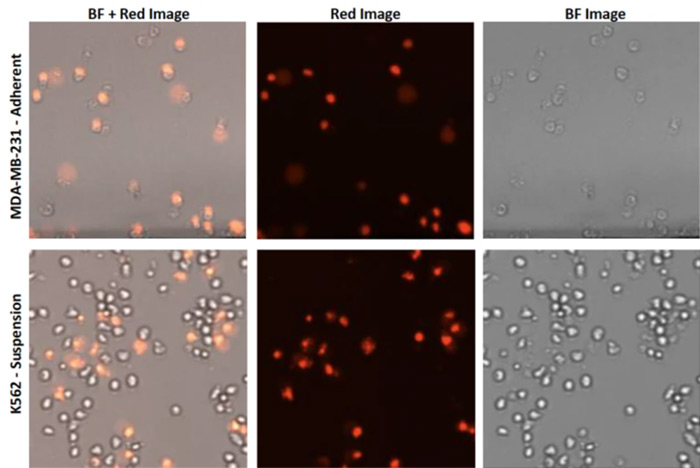
Example images of MDA-MB-231 and K562 cells treated with 25 µm Benzethonium for 24h and stained with PI.
These research studies show that repurposing existing drugs is a promising avenue for multiple investigative areas of vital importance to public health. This includes the ever-present threat of cancer, as well as the current Coronavirus pandemic plaguing the world. The studies also demonstrate that many candidates already exist within the world’s expansive pharmacopeia that can be evaluated for anti-virus or anti-cancer activity. The success of these experiments shows the potential of applying old solutions against new problems.
Learn more about modern virology assays by visiting our virology web page»
References:
- https://www.nature.com/articles/d41586-020-02926-w
- https://www.who.int/news/item/15-07-2020-more-than-150-countries-engaged-in-covid-19-vaccine-global-access-facility/
- https://www.nature.com/articles/s41586-020-2286-9
- https://pubmed.ncbi.nlm.nih.gov/32707573/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4972561/
- https://pubmed.ncbi.nlm.nih.gov/30102978/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5797010/






Leave A Comment