Detection of novel CD276-CAR NK-92 mediated cytotoxicity of neuroblastoma tumor spheroids using plate-based image cytometry.
Neuroblastoma impact demands new therapeutic approaches
Frequent relapses, severe side effects, and high mortality rates amongst high-risk pediatric patients with rare cancerous neuroblastoma (NB) necessitate new therapeutic approaches. Many factors limit this advancement including cost-efficiency issues, extensive process limitations of autologous CAR products, and manufacturing scalability issues. Learning from the most current and advanced methods and technologies is the only way to break through these roadblocks.
CAR-engineering therapeutic targets for neuroblastomas
Recent studies show chimeric antigen receptor (CAR)-modified immune cells can elicit specific clinical response targeting of CD19 and CD22 in hematological malignancies. Utilizing specialized CAR-engineering, type I transmembrane human protein encoded CD276-CAR NK-92, can directly target neuroblastoma. Researchers in this study “generated CAR-engineered NK-92 cells which successfully eliminate NB cell lines in monolayer cultures as well as 3D models in vitro by targeting the pan-cancer antigen CD276.”1
3D multicellular neuroblastoma spheroids
Three-dimensional (3D) spheroid culture models have been shown to improve clinical translation due to better physiological relevance compared to cell monolayer culture systems. The utilization of 3D multicellular NB spheroids to mimic 3D tumor structure greatly supports the investigation of intratumoral cytotoxicity efficacy of the CD276-CAR NK-92 cells.
Neuroblastoma spheroid cytotoxicity assay for rapid metrics
Researchers employed the Celigo Imaging Cytometer to monitor spheroid growth as well as integrated fluorescent intensity. GFP-positive NB cells were seeded into Nexcelom3D 96-well ultra-low adhesion round-bottom plates. Spheroids were grown for 72 hours then co-incubated with CD276-CAR NK-92 or native NK-92 cells to determine whether CAR signaling could lyse the NB cells. Fluorescence was measured at the time points indicated in Figure 1 over 96 hours. CAR NK-mediated cytotoxicity was effortlessly calculated using Celigo metrics for average integrated fluorescence intensity of NB spheroids.
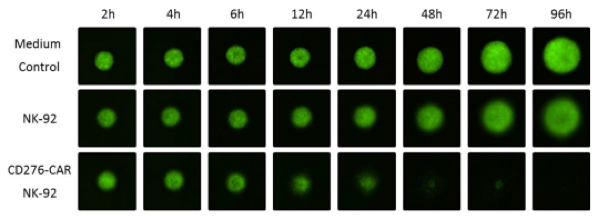
Figure 1 Integrated fluorescence intensity of neuroblastoma Spheroids rapidly measured with the Celigo S Imaging Cytometer
Removing CAR cell side effects from the equation
Measuring CAR NK-mediated cytotoxicity is important as CAR T cells can induce severe side effects, including cytokine release syndrome (CRS) and neurotoxicity due to “on-target, off-tumor toxicity.” This research shows the potential for newer, more accurate, and highly direct treatment options for high-risk neuroblastoma with potentially lower side effects.
Please visit our applications page for more information on how to conduct cell-mediated cytotoxicity assays and 3D assays using the Celigo Imaging Cytometer.
References
Grote, S, Chan, KC‐H, Baden, C, et al. CD276 as a novel CAR NK‐92 therapeutic target for neuroblastoma. Adv Cell Gene Ther. 2020; 00:e105. https://doi.org/10.1002/acg2.105






Leave A Comment